Question: instructions below all the information needed in given below. please explain as much as possible 5. Molecular Formula: C13Hz 'H NMR 6 1.65 ppm, 1H,
instructions below all the information needed in given below. please explain as much as possible
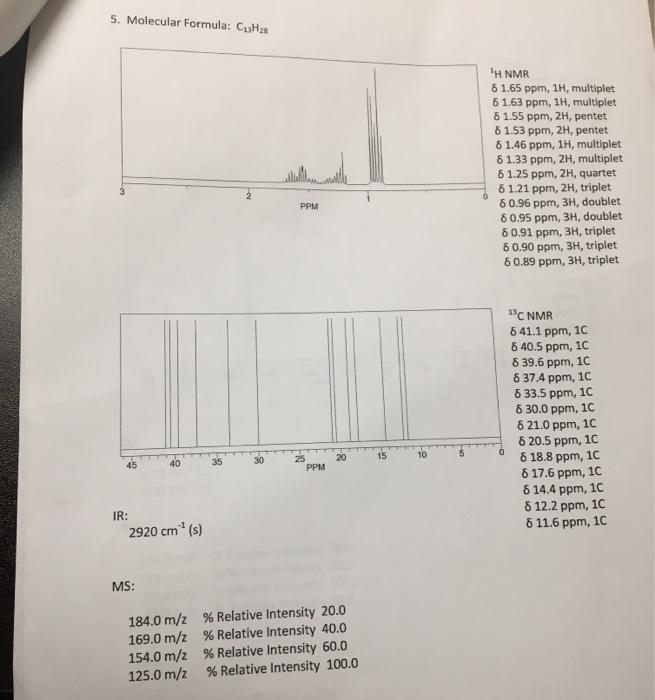

5. Molecular Formula: C13Hz 'H NMR 6 1.65 ppm, 1H, multiplet 61.63 ppm, 1H, multiplet 61.55 ppm, 2H, pentet 61.53 ppm, 2H, pentet 8 1.46 ppm, 1H, multiplet 61.33 ppm, 2H, multiplet 6 1.25 ppm, 2H, quartet 61.21 ppm, 2H, triplet 6 0.96 ppm, 3H, doublet 60.95 ppm, 3H, doublet 80.91 ppm, 3H, triplet 6 0.90 ppm, 3H, triplet 80.89 ppm, 3H, triplet PPM 13C NMR 641.1 ppm, 10 8 40.5 ppm, 1C 8 39.6 ppm, 10 8 37.4 ppm, 1C 633.5 ppm, 1C 6 30.0 ppm, 1C 6 21.0 ppm, 1C 6 20.5 ppm, 10 8 18.8 ppm, 1C 617.6 ppm, 6 14.4 ppm, 1C 6 12.2 ppm, 1C 8 11.6 ppm, 10 10 5 20 15 30 45 35 40 25 PPM 1C IR: 2920 cm (s) MS: 184.0 m/z % Relative Intensity 20.0 169.0 m/z % Relative Intensity 40.0 154.0 m/z % Relative Intensity 60.0 125.0 m/z % Relative Intensity 100.0 Instructions For each question: 1. Provide a chemical structure and appropriate IUPAC name for the compound 2. Interpret the 'H NMR (you should identify specifically each peak in the spectrum as to which hydrogen(s) that peak corresponds to on the spectrum) 3. Interpret the "C NMR (you should identify specifically each peak in the spectrum as to which carbon(s) that peak corresponds to on the spectrum) 4. Specifically identify each absorption in the IR spectrum (ie. C-H stretch or C=0 stretch, etc.) 5. Provide structural fragments for each m/z in the mass spectrum (remember that these fragments are charged). Do not just give the molecular formula, please give the actual structure of the fragment
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


