Question: Intro: Cyclopropane molecules are often used as radical clocks or traps in reaction mechanism experiments. Essentially, if a radical is present in a mechanism, the
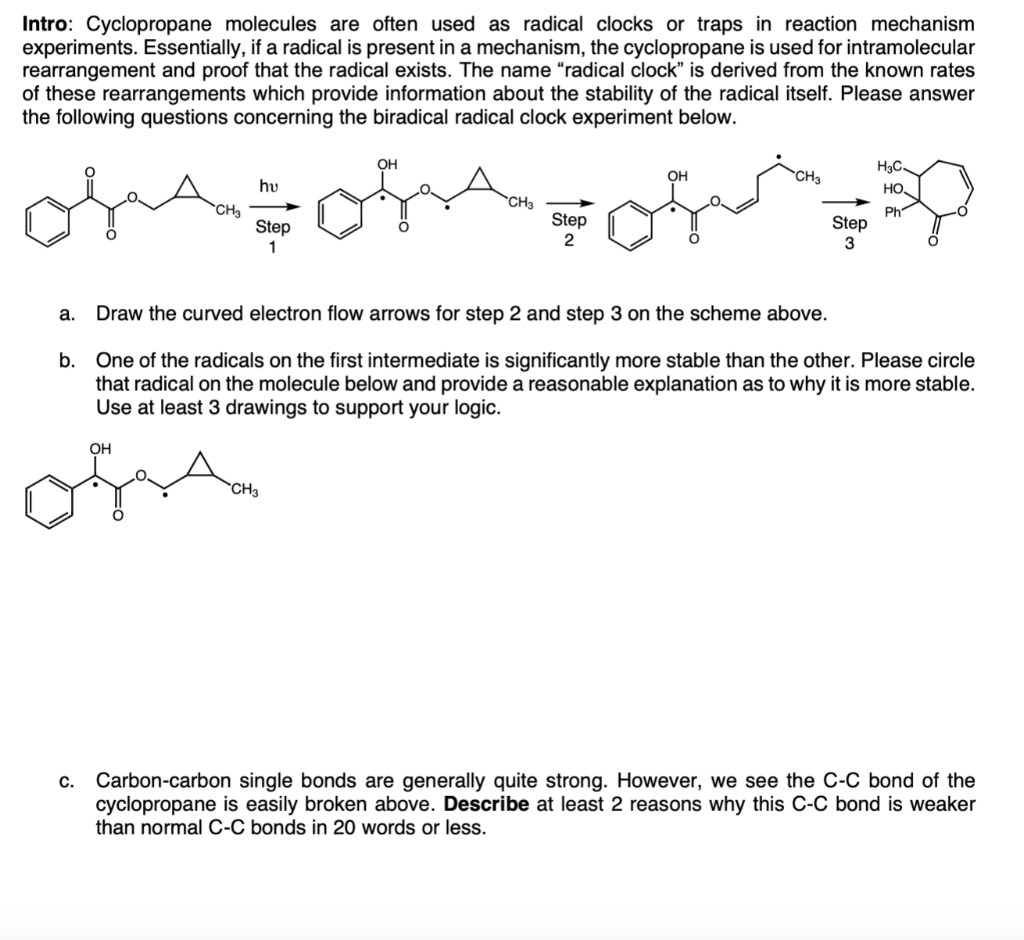
Intro: Cyclopropane molecules are often used as radical clocks or traps in reaction mechanism experiments. Essentially, if a radical is present in a mechanism, the cyclopropane is used for intramolecular rearrangement and proof that the radical exists. The name "radical clock" is derived from the known rates of these rearrangements which provide information about the stability of the radical itself. Please answer the following questions concerning the biradical radical clock experiment below. 3 a. Draw the curved electron flow arrows for step 2 and step 3 on the scheme above. b. One of the radicals on the first intermediate is significantly more stable than the other. Please circle that radical on the molecule below and provide a reasonable explanation as to why it is more stable. Use at least 3 drawings to support your logic. c. Carbon-carbon single bonds are generally quite strong. However, we see the C-C bond of the cyclopropane is easily broken above. Describe at least 2 reasons why this CC bond is weaker than normal CC bonds in 20 words or less
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


