Question: Introduction to Galvanic Cells Learning Goal: To understand the components and processes of a galvanic cell. A galvanic cell (or voltaic cell) produces electricity
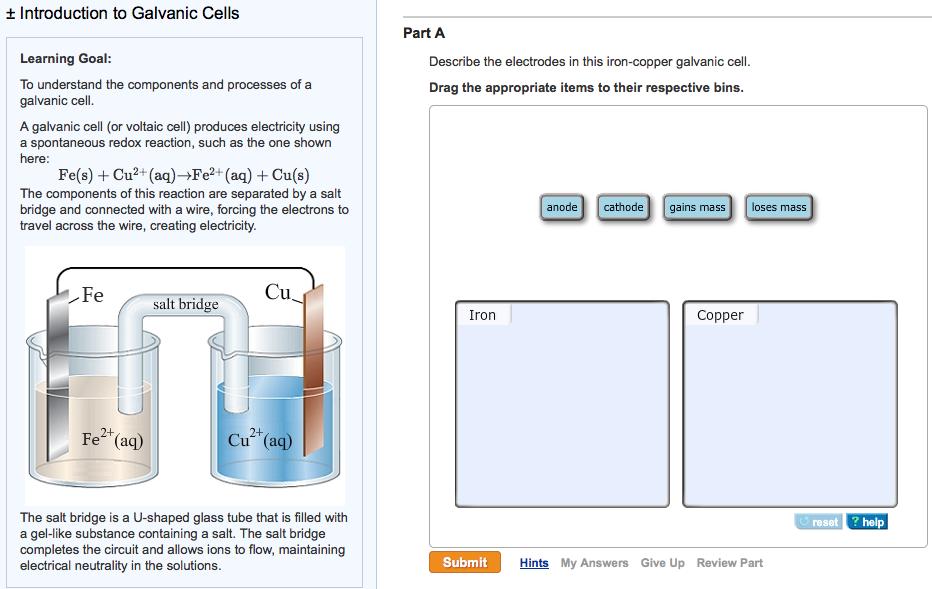
Introduction to Galvanic Cells Learning Goal: To understand the components and processes of a galvanic cell. A galvanic cell (or voltaic cell) produces electricity using a spontaneous redox reaction, such as the one shown here: Fe(s) + Cu+ (aq) Fe+ (aq) + Cu(s) The components of this reaction are separated by a salt bridge and connected with a wire, forcing the electrons to travel across the wire, creating electricity. Fe Fe+ (aq) salt bridge Cu 2+ Cu+ (aq) The salt bridge is a U-shaped glass tube that is filled with a gel-like substance containing a salt. The salt bridge completes the circuit and allows ions to flow, maintaining electrical neutrality in the solutions. Part A Describe the electrodes in this iron-copper galvanic cell. Drag the appropriate items to their respective bins. Iron Submit anode cathode gains mass Copper loses mass Hints My Answers Give Up Review Part reset ? help
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
SOLUTION 1 a Tin is an anode and it looses its ... View full answer

Get step-by-step solutions from verified subject matter experts


