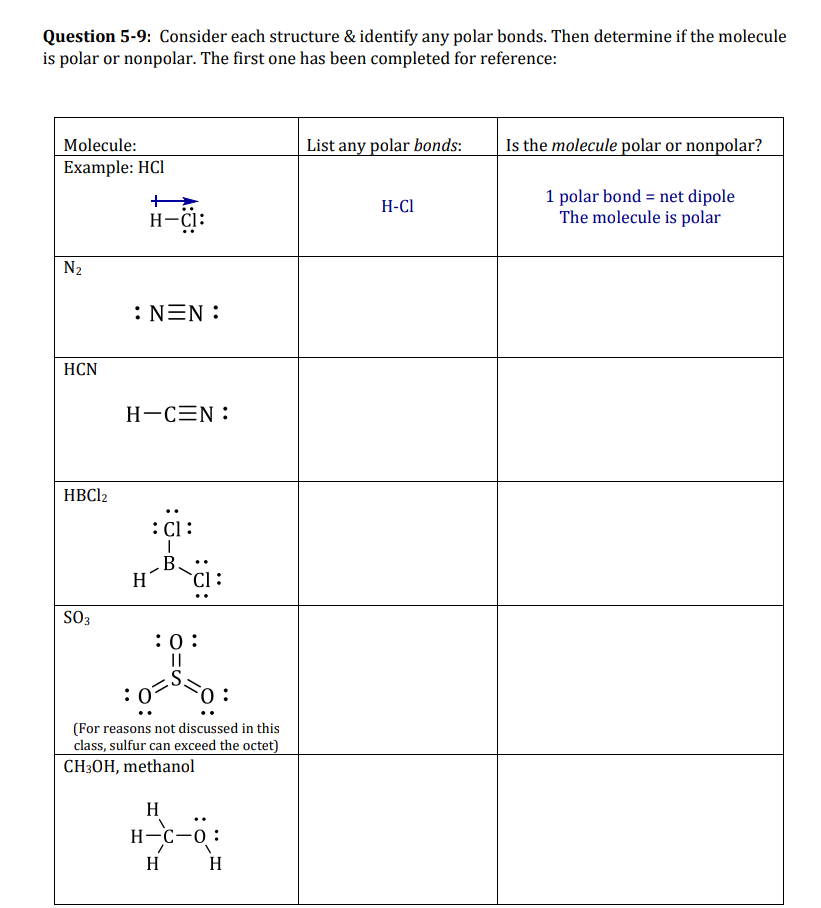
Question: is urgent pls Question 5-9: Consider each structure & identify any polar bonds. Then determine if the molecule is polar or nonpolar. The first one
is urgent pls
Question 5-9: Consider each structure & identify any polar bonds. Then determine if the molecule is polar or nonpolar. The first one has been completed for reference: List any polar bonds: Is the molecule polar or nonpolar? Molecule: Example: HCI H-C1 1 polar bond = net dipole The molecule is polar H-CI: N2 :NEN: HCN H-CEN: HBCl2 :C: B H Cl: SO3 :0: :0-5507 : : (For reasons not discussed in this class, sulfur can exceed the octet) CH3OH, methanol .. H H-C-0: --o H H HC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


