Question: It a certain temperature, the given reaction has an equilibrium constant of Kp=327. PCl3(g)+Cl2(g)PCl5(g) PCl5 is placed in a sealed container at an initial pressure
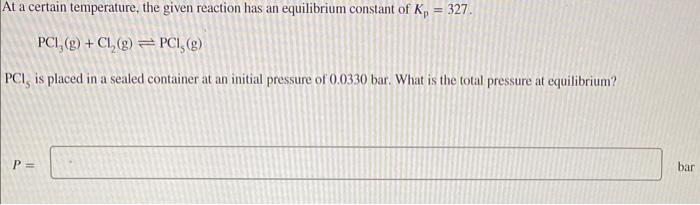
It a certain temperature, the given reaction has an equilibrium constant of Kp=327. PCl3(g)+Cl2(g)PCl5(g) PCl5 is placed in a sealed container at an initial pressure of 0.0330 bar. What is the total pressure at equilibrium? P=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


