Question: it is continue to this question 1. Calculate the bottoms composition again analytically, but instead of assuming relative volatility (RV)=5, use the equilibrium curve given.

it is continue to this question
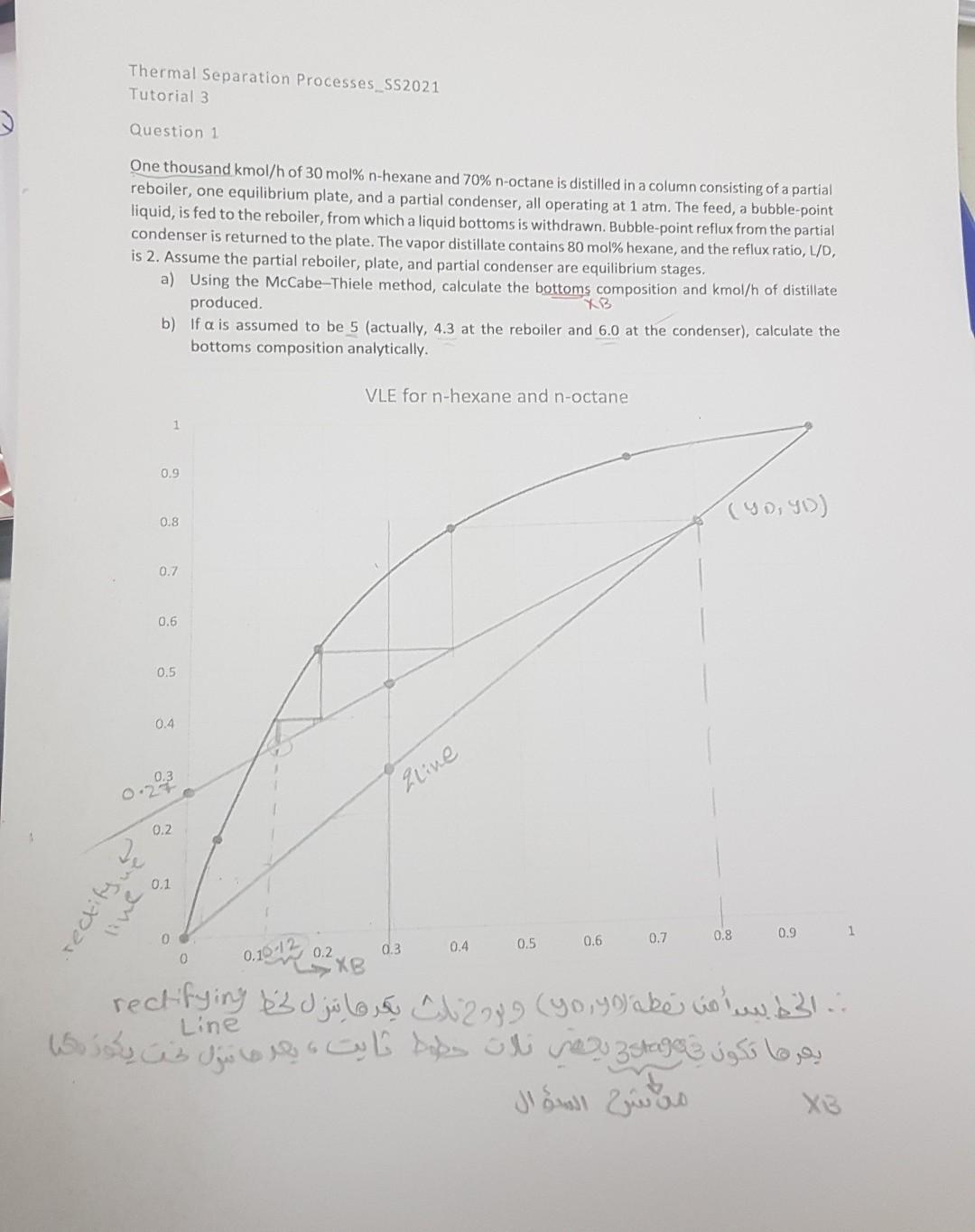
1. Calculate the bottoms composition again analytically, but instead of assuming relative volatility (RV)=5, use the equilibrium curve given. (Note: You're required to solve the problem analytically, so use the equilibrium curve as needed, but not the Mc Cabe Thiele Method.) 2. According to your understanding, explain why the RV at the condenser is greater than that at the bottom (reboiler)? Would it make sense for it to be the opposite? Tutorial 3 Question 1 One thousand kmol/h of 30mol%n-hexane and 70%n-octane is distilled in a column consisting of a partial reboiler, one equilibrium plate, and a partial condenser, all operating at 1atm. The feed, a bubble-point liquid, is fed to the reboiler, from which a liquid bottoms is withdrawn. Bubble-point reflux from the partial condenser is returned to the plate. The vapor distillate contains 80 mol\% hexane, and the reflux ratio, L/D, is 2. Assume the partial reboiler, plate, and partial condenser are equilibrium stages. a) Using the McCabe-Thiele method, calculate the bottoms composition and kmol/h of distillate produced. b) If is assumed to be 5 (actually, 4.3 at the reboiler and 6.0 at the condenser), calculate the bottoms composition analytically. Josil 2 juso
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


