Question: It is urgent guys. Can u help ?? The number of molecules of a monoatomical ideal gas is N and is constant. In all processes,
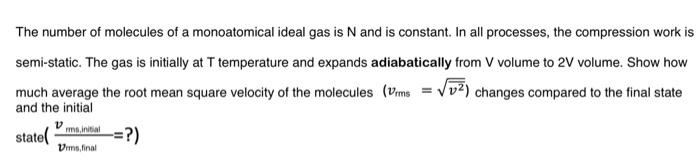
The number of molecules of a monoatomical ideal gas is N and is constant. In all processes, the compression work is semi-static. The gas is initially at T temperature and expands adiabatically from V volume to 2V volume. Show how much average the root mean square velocity of the molecules (vrms=v2) changes compared to the final state and the initial state(vmb,finalvmstinital=?) The number of molecules of a monoatomical ideal gas is N and is constant. In all processes, the compression work is semi-static. The gas is initially at T temperature and expands adiabatically from V volume to 2V volume. Show how much average the root mean square velocity of the molecules (vrms=v2) changes compared to the final state and the initial state(vmb,finalvmstinital=?)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


