Question: It's Che 321 class. Please help me if you know how to do it. I will rate it up. Don't solve it by putting in
It's Che 321 class. Please help me if you know how to do it. I will rate it up. Don't solve it by putting in some wrong answers. I will rate it down. Thank you.
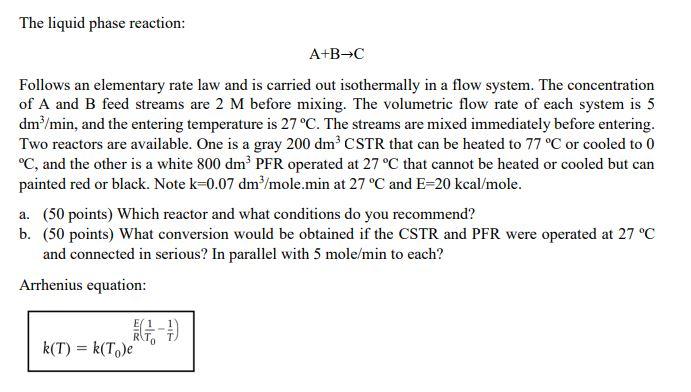
The liquid phase reaction: A+BC Follows an elementary rate law and is carried out isothermally in a flow system. The concentration of A and B feed streams are 2M before mixing. The volumetric flow rate of each system is 5 dm3/min, and the entering temperature is 27C. The streams are mixed immediately before entering. Two reactors are available. One is a gray 200dm3CSTR that can be heated to 77C or cooled to 0 C, and the other is a white 800dm3 PFR operated at 27C that cannot be heated or cooled but can painted red or black. Note k=0.07dm3/ mole.min at 27C and E=20kcal/mole. a. (50 points) Which reactor and what conditions do you recommend? b. (50 points) What conversion would be obtained if the CSTR and PFR were operated at 27C and connected in serious? In parallel with 5mole/min to each? Arrhenius equation: k(T)=k(T0)eR^E(T01T1)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


