Question: it's combustion class 1) Estimate the laminar flame speed for stoichiometric methane/air combustion. Use the global one-step reaction mechanism (Equation 5.2 and Table 5.1) to
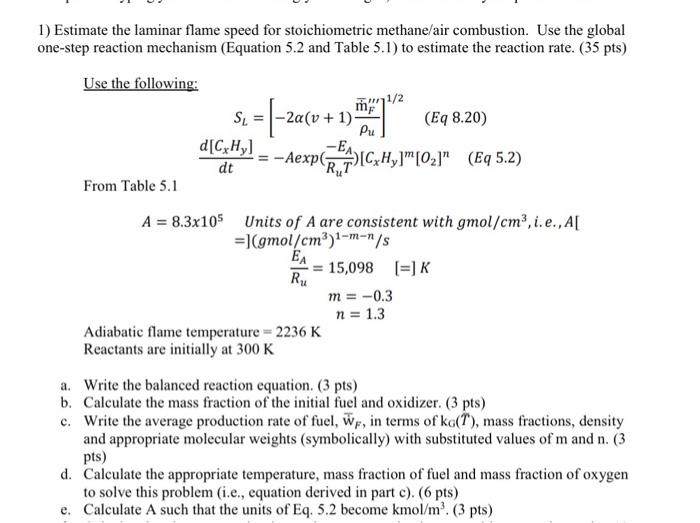
1) Estimate the laminar flame speed for stoichiometric methane/air combustion. Use the global one-step reaction mechanism (Equation 5.2 and Table 5.1) to estimate the reaction rate. (35 pts) Use the following: 1/2 SL (Eq 8.20) Pu d[CxHy] dt From Table 5.1 =(-2a(v + 1) -Aexpez ICH,TM10.5" (q 5.2) Ru A = 8.3x105 Units of A are consistent with gmol/cm, i.e., A[ = (gmol/cm3)1-m-/s EA = 15,098 [=] K m = -0.3 n = 1.3 Adiabatic flame temperature = 2236 K Reactants are initially at 300 K a. Write the balanced reaction equation. (3 pts) b. Calculate the mass fraction of the initial fuel and oxidizer. (3 pts) c. Write the average production rate of fuel, wr, in terms of ko(1), mass fractions, density and appropriate molecular weights (symbolically) with substituted values of m and n. (3 pts) d. Calculate the appropriate temperature, mass fraction of fuel and mass fraction of oxygen to solve this problem (i.e., equation derived in part c). (6 pts) e. Calculate A such that the units of Eq. 5.2 become kmol/m (3 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


