Question: its not A or neither, ive checked and its both wrong. pls help Two solutions are created by mixing one solution containing calcium bromide with
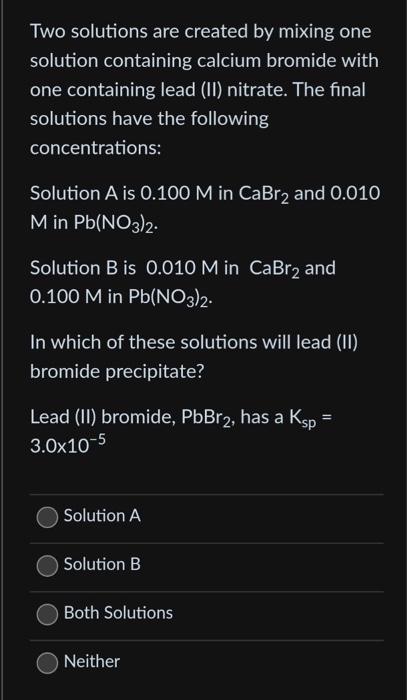
Two solutions are created by mixing one solution containing calcium bromide with one containing lead (II) nitrate. The final solutions have the following concentrations: Solution A is 0.100M in CaBr2 and 0.010 M in Pb(NO3)2. Solution B is 0.010M in CaBr2 and 0.100M in Pb(NO3)2. In which of these solutions will lead (II) bromide precipitate? Lead (II) bromide, PbBr2, has a Ksp= 3.0105 Solution A Solution B Both Solutions Neither
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


