Question: iv- A gas mixture containing 21%O2 and 79%N2 flows through a pipe of circular cross-section of diameter 2cm at atmospheric pressure. The velocity of O2
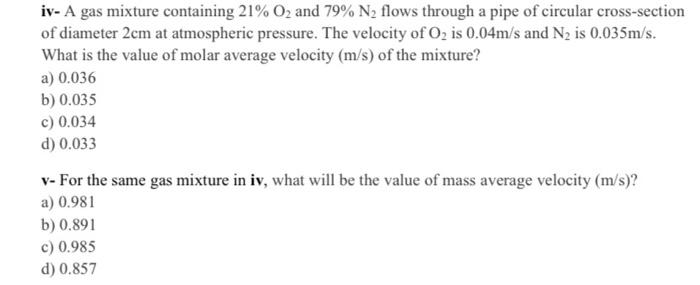
iv- A gas mixture containing 21%O2 and 79%N2 flows through a pipe of circular cross-section of diameter 2cm at atmospheric pressure. The velocity of O2 is 0.04m/s and N2 is 0.035m/s. What is the value of molar average velocity (m/s) of the mixture? a) 0.036 b) 0.035 c) 0.034 d) 0.033 v- For the same gas mixture in iv, what will be the value of mass average velocity (m/s) ? a) 0.981 b) 0.891 c) 0.985 d) 0.857
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


