Question: just need all the propagated errors Table 1A Averege densitv/ standard deviation and proparated errors. begin{tabular}{|r|r|r|r|r|r|} multicolumn{2}{|c|}{ Burette } & multicolumn{2}{c|}{ Pipette } & multicolumn{2}{c|}{
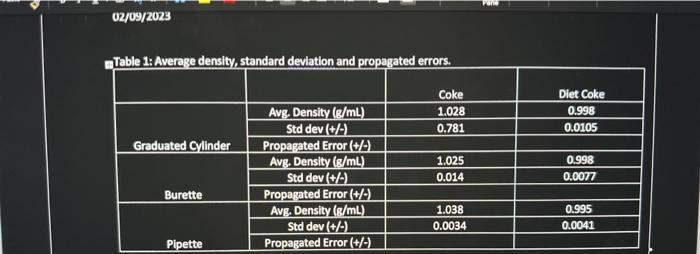
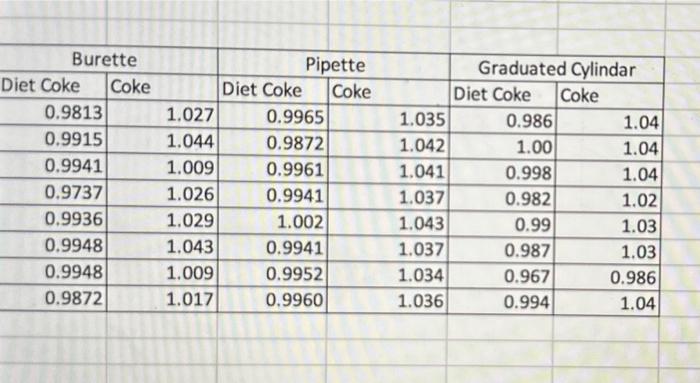
Table 1A Averege densitv/ standard deviation and proparated errors. \begin{tabular}{|r|r|r|r|r|r|} \multicolumn{2}{|c|}{ Burette } & \multicolumn{2}{c|}{ Pipette } & \multicolumn{2}{c|}{ Graduated Cylindar } \\ \hline Diet Coke & \multicolumn{1}{|l|}{ Coke } & \multicolumn{1}{c|}{ Diet Coke } & Coke & \multicolumn{1}{c|}{ Diet Coke } & \multicolumn{1}{l|}{ Coke } \\ \hline 0.9813 & 1.027 & 0.9965 & 1.035 & 0.986 & 1.04 \\ \hline 0.9915 & 1.044 & 0.9872 & 1.042 & 1.00 & 1.04 \\ \hline 0.9941 & 1.009 & 0.9961 & 1.041 & 0.998 & 1.04 \\ \hline 0.9737 & 1.026 & 0.9941 & 1.037 & 0.982 & 1.02 \\ \hline 0.9936 & 1.029 & 1.002 & 1.043 & 0.99 & 1.03 \\ \hline 0.9948 & 1.043 & 0.9941 & 1.037 & 0.987 & 1.03 \\ \hline 0.9948 & 1.009 & 0.9952 & 1.034 & 0.967 & 0.986 \\ \hline 0.9872 & 1.017 & 0.9960 & 1.036 & 0.994 & 1.04 \\ \hline \end{tabular} Table 1A Averege densitv/ standard deviation and proparated errors. \begin{tabular}{|r|r|r|r|r|r|} \multicolumn{2}{|c|}{ Burette } & \multicolumn{2}{c|}{ Pipette } & \multicolumn{2}{c|}{ Graduated Cylindar } \\ \hline Diet Coke & \multicolumn{1}{|l|}{ Coke } & \multicolumn{1}{c|}{ Diet Coke } & Coke & \multicolumn{1}{c|}{ Diet Coke } & \multicolumn{1}{l|}{ Coke } \\ \hline 0.9813 & 1.027 & 0.9965 & 1.035 & 0.986 & 1.04 \\ \hline 0.9915 & 1.044 & 0.9872 & 1.042 & 1.00 & 1.04 \\ \hline 0.9941 & 1.009 & 0.9961 & 1.041 & 0.998 & 1.04 \\ \hline 0.9737 & 1.026 & 0.9941 & 1.037 & 0.982 & 1.02 \\ \hline 0.9936 & 1.029 & 1.002 & 1.043 & 0.99 & 1.03 \\ \hline 0.9948 & 1.043 & 0.9941 & 1.037 & 0.987 & 1.03 \\ \hline 0.9948 & 1.009 & 0.9952 & 1.034 & 0.967 & 0.986 \\ \hline 0.9872 & 1.017 & 0.9960 & 1.036 & 0.994 & 1.04 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


