Question: just need some help with figuring this out. im not sure what its asking me to do. Atoms join or bond together to form molecules.
just need some help with figuring this out. im not sure what its asking me to do.
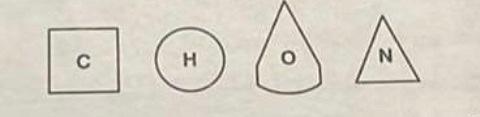
Atoms join or bond together to form molecules. Within these molecules the atoms are held together by various chemical bonds. One type of chemical bond is the covalent bond, in which electrons in the outer orbital shell are shared. For understanding and drawing covalent chemical bonds, various shapes will be used here for the elements carbon, hydrogen, oxygen, and nitrogen, as follows.
- By drawing the shapes attached to one another, you can indicate chemical bonds being formed between the represented atoms. Some of the bonds you construct will be single bonds, in which only one electron from each atom is shared. Some will be double bonds, in which two electrons from each atom are shared. To indicate single bonds, draw the shapes attached by the sides. Show double bonds by attaching the shapes by the corners. (In showing bonds, - is single; = is double.)
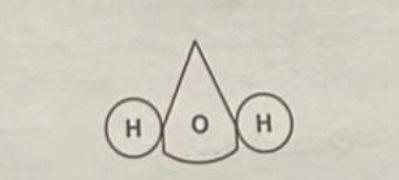
- In water, as an example, three atoms are found in one molecule: one oxygen and two hydrogens. A molecule of water drawn using the reference shapes is as follows.
- The structural formula for the water moleculeis: O-H
- The molecular formula for the water molecule is: H2O
C. H
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


