Question: just the answer no explanation needed 6. A 0.19 M solution of a weak acid HA is 3.30 % ionized. What are the H.A, and
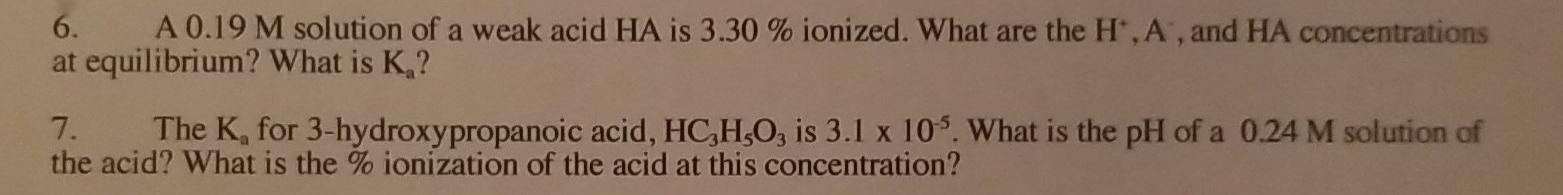
just the answer no explanation needed
6. A 0.19 M solution of a weak acid HA is 3.30 % ionized. What are the H.A, and HA concentrations at equilibrium? What is K,? 7. The K, for 3-hydroxypropanoic acid, HC,H,O, is 3.1 x 105. What is the pH of a 0.24 M solution of the acid? What is the % ionization of the acid at this concentration
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


