Question: just the material balance part ,using 100 as a basis for stream 7 Consider the process flowsheet for Ammonia production depicted below where ammonia is
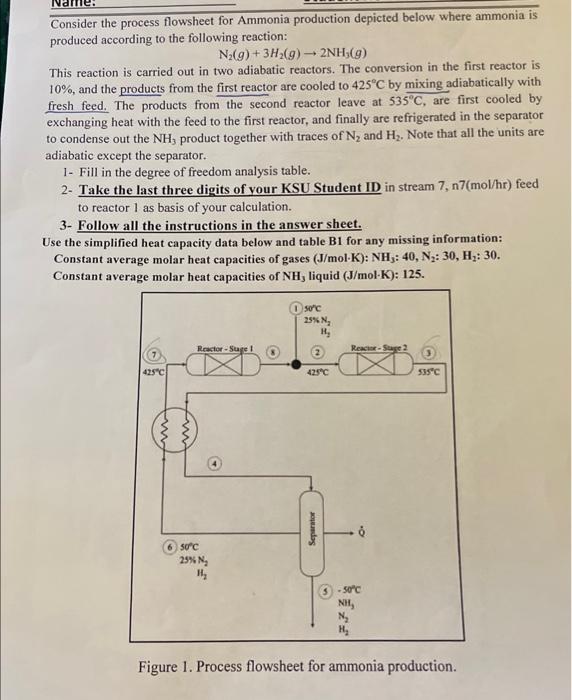
Consider the process flowsheet for Ammonia production depicted below where ammonia is produced according to the following reaction: N2(g)+3H2(g)2NH3(g) This reaction is carried out in two adiabatic reactors. The conversion in the first reactor is 10%, and the products from the first reactor are cooled to 425C by mixing adiabatically with fresh feed. The products from the second reactor leave at 535C, are first cooled by exchanging heat with the feed to the first reactor, and finally are refrigerated in the separator to condense out the NH3 product together with traces of N2 and H2. Note that all the units are adiabatic except the separator. 1- Fill in the degree of freedom analysis table. 2- Take the last three digits of your KSU Student ID in stream 7,n7(mol/hr) feed to reactor 1 as basis of your calculation. 3- Follow all the instructions in the answer sheet. Use the simplified heat capacity data below and table B1 for any missing information: Constant average molar heat capacities of gases (J/molK):NH3:40,N2:30,H2:30. Constant average molar heat capacities of NH3 liquid (J/molK):125. Figure 1. Process flowsheet for ammonia production
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


