Question: k 0 HOCEl_(3) +HI +H_(2)O a= Proton transfer e= Electrophilic addition h=S_(N)1 Nucleophilic substitution b= Lewis acid/base f=E1 Elimination i=S_(N)2 Nucleophilic
k
0\
HOCEl_(3)\
+HI\
+H_(2)O\
a=Proton transfer\
e=Electrophilic addition\
h=S_(N)1Nucleophilic substitution\
b=Lewis acid/base\
f=E1Elimination\
i=S_(N)2Nucleophilic substitution\
g=E2 Elimination\ j = Electrophilic aromatic substitution\ Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters
a-
jfor your answers.\ 1.\ 2.\ 1 more group attempt remaining
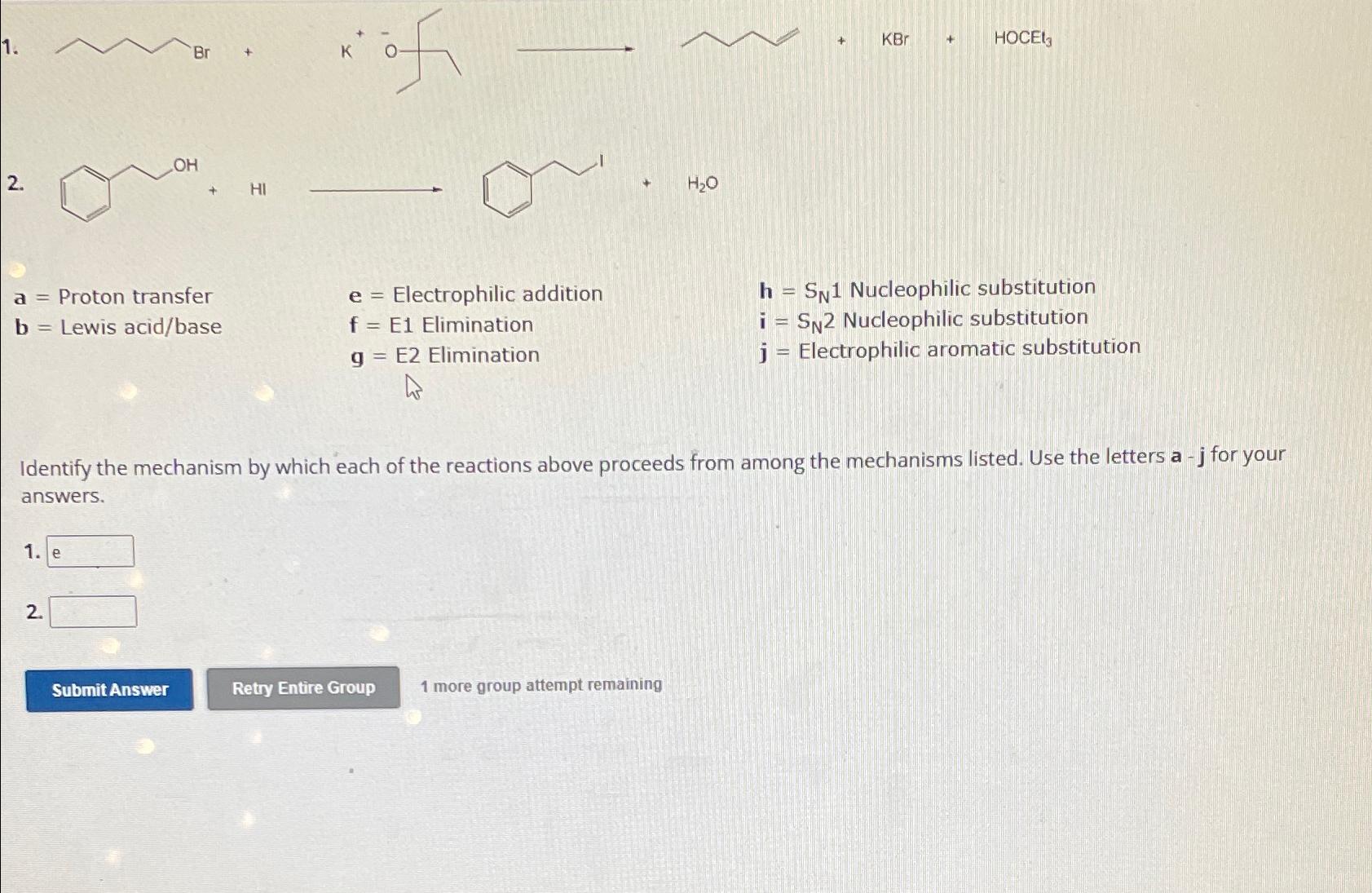
2. e= Electrophilic addition f=E1 Elimination g=E2 Elimination h=SN1 Nucleophilic substitution i=SN2 Nucleophilic substitution j = Electrophilic aromatic substitution a= Proton transfer b= Lewis acid/base +H2O j = Electrophin aromatic substitution Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters aj for your answers. 1. 2. 1 more group attempt remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


