Question: keep plugging in and getting wrong answers If the rate constant for the decomposition reaction of cyclobutane into ethylene is 2.08102s1 at a certain temperature,
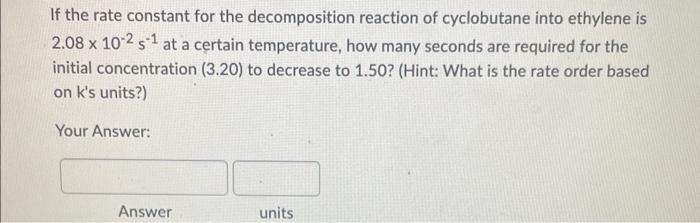
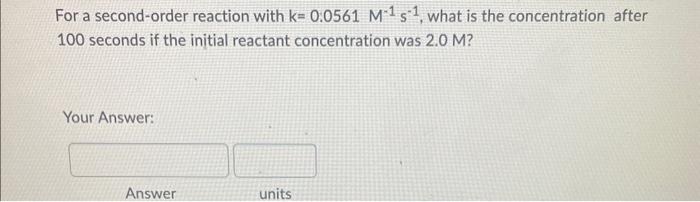
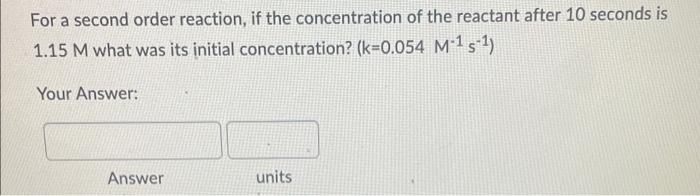
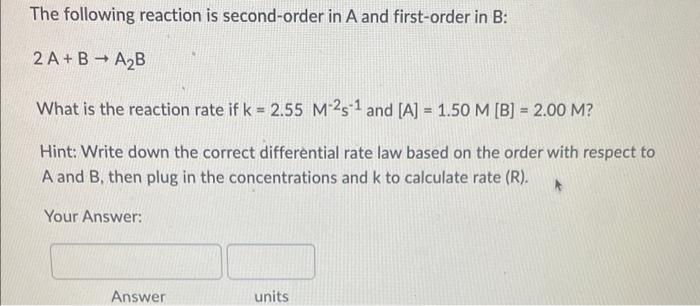
If the rate constant for the decomposition reaction of cyclobutane into ethylene is 2.08102s1 at a certain temperature, how many seconds are required for the initial concentration (3.20) to decrease to 1.50? (Hint: What is the rate order based on k's units?) Your Answer: For a second-order reaction with k=0.0561M1s1, what is the concentration after 100 seconds if the initial reactant concentration was 2.0M ? Your Answer: For a second order reaction, if the concentration of the reactant after 10 seconds is 1.15M what was its initial concentration? (k=0.054M1s1) Your Answer: Answer units The following reaction is second-order in A and first-order in B: 2A+BA2B What is the reaction rate if k=2.55M2s1 and [A]=1.50M[B]=2.00M ? Hint: Write down the correct differential rate law based on the order with respect to A and B, then plug in the concentrations and k to calculate rate (R). Your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


