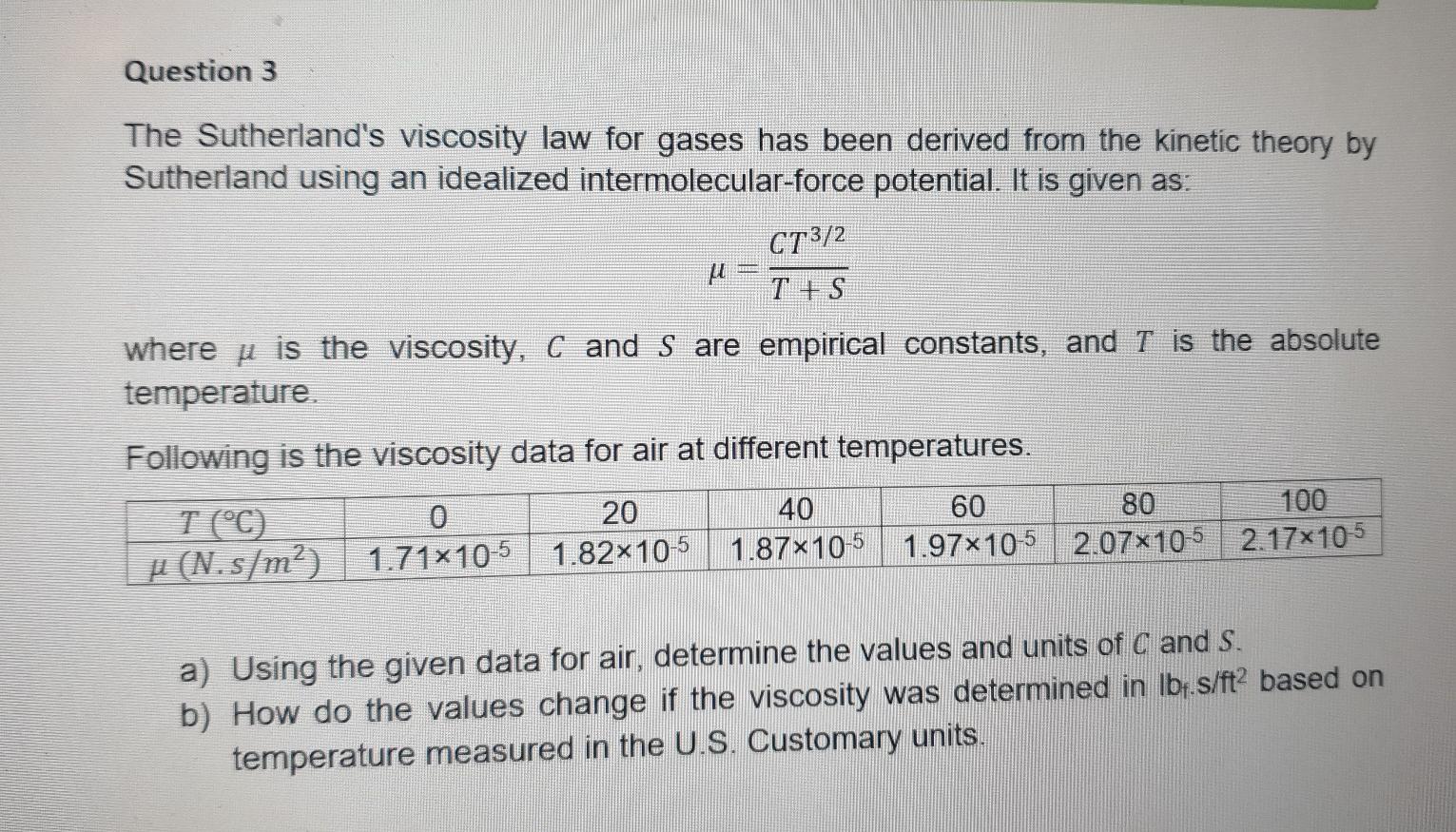
Question: kindly answer a & b Question 3 The Sutherland's viscosity law for gases has been derived from the kinetic theory by Sutherland using an idealized
kindly answer a & b
Question 3 The Sutherland's viscosity law for gases has been derived from the kinetic theory by Sutherland using an idealized intermolecular-force potential. It is given as: CT3/2 U T + S where u is the viscosity, C and S are empirical constants, and T is the absolute temperature Following is the viscosity data for air at different temperatures. T (C) 20 40 60 80 100 u (N.s/m?) 1.71x10-5 1.82x10-5 1.87x10-5 1.97x10-5 2.07x10-5 2.17x105 a) Using the given data for air, determine the values and units of C and S. b) How do the values change if the viscosity was determined in lbf.s/ft2 based on temperature measured in the U.S. Customary units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


