Question: Kindly answer these. thanks ! #6: A student is conducting molecular weight determination of an unknown diprotic acid. He first prepared a 250 ml NaOH
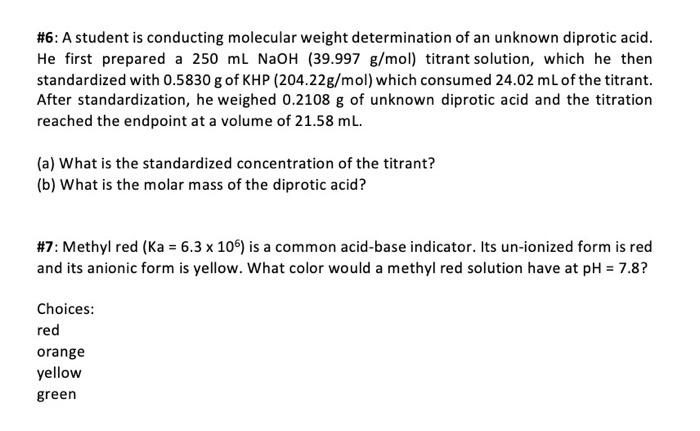
#6: A student is conducting molecular weight determination of an unknown diprotic acid. He first prepared a 250 ml NaOH (39.997 g/mol) titrant solution, which he then standardized with 0.5830 g of KHP (204.22g/mol) which consumed 24.02 mL of the titrant. After standardization, he weighed 0.2108 g of unknown diprotic acid and the titration reached the endpoint at a volume of 21.58 ml. (a) What is the standardized concentration of the titrant? (b) What is the molar mass of the diprotic acid? #7: Methyl red (Ka = 6.3 x 106) is a common acid-base indicator. Its un-ionized form is red and its anionic form is yellow. What color would a methyl red solution have at pH = 7.8? Choices: red orange yellow green
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


