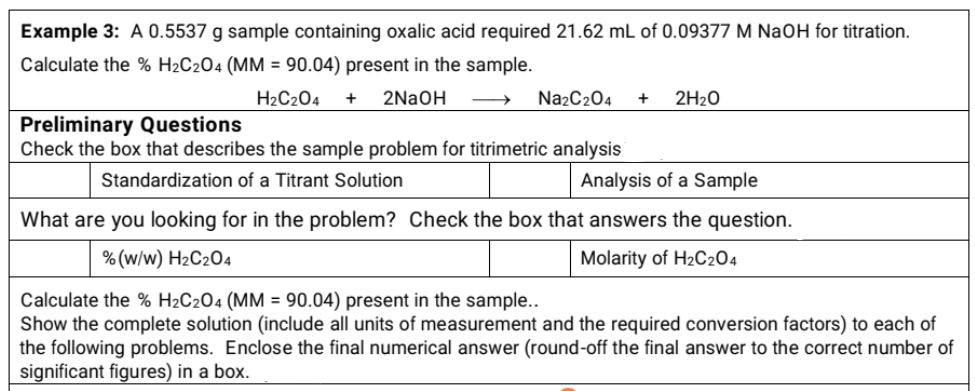
Question: Kindly answer this ASAP! Example 3: A 0.5537g sample containing oxalic acid required 21.62mL of 0.09377MNaOH for titration. Calculate the %H2C2O4(MM=90.04) present in the sample.
 Kindly answer this ASAP!
Kindly answer this ASAP!
Example 3: A 0.5537g sample containing oxalic acid required 21.62mL of 0.09377MNaOH for titration. Calculate the %H2C2O4(MM=90.04) present in the sample. H2C2O4+2NaOHNa2C2O4+2H2O Preliminary Questions Check the box that describes the sample problem for titrimetric analysis \begin{tabular}{|l|l|l|l|} \hline & Standardization of a Titrant Solution & & Analysis of a Sample \\ \hline \end{tabular} What are you looking for in the problem? Check the box that answers the question. \begin{tabular}{|l|l|l|l|} \hline & %(w/w)H2C2O4 & & Molarity of H2C2O4 \\ \hline \end{tabular} Calculate the %H2C2O4(MM=90.04) present in the sample.. Show the complete solution (include all units of measurement and the required conversion factors) to each of the following problems. Enclose the final numerical answer (round-off the final answer to the correct number of significant figures) in a box
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


