Question: Kindly give the correct answer I will upvote. A mixture of fuel containing 0.550 mol CH /mol, 0.350 mol C2H,/mol, and 0.100 mol C /2/mol
Kindly give the correct answer I will upvote.
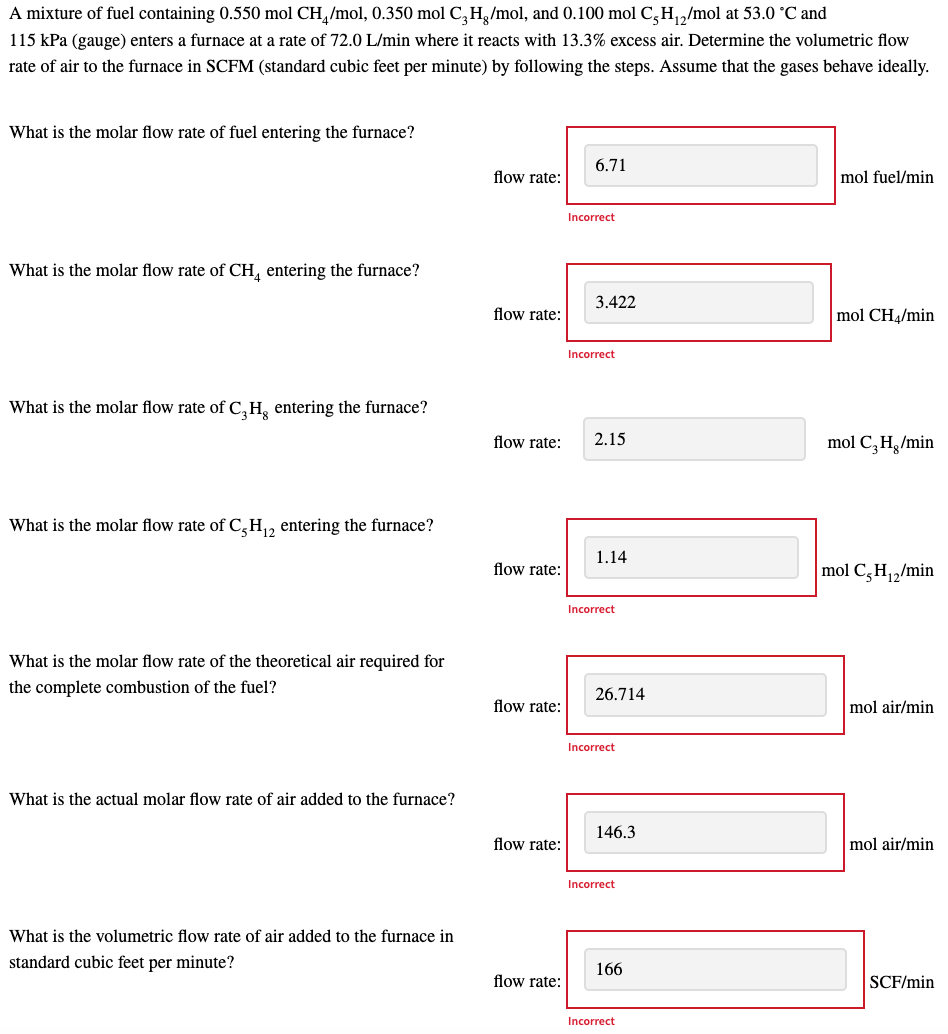
A mixture of fuel containing 0.550 mol CH /mol, 0.350 mol C2H,/mol, and 0.100 mol C /2/mol at 53.0 C and 115 kPa (gauge) enters a furnace at a rate of 72.0 L/min where it reacts with 13.3% excess air. Determine the volumetric flow rate of air to the furnace in SCFM (standard cubic feet per minute) by following the steps. Assume that the gases behave ideally. What is the molar flow rate of fuel entering the furnace? 6.71 flow rate: mol fuel/min Incorrect What is the molar flow rate of CH, entering the furnace? 3.422 flow rate: mol CH4/min Incorrect What is the molar flow rate of C,H, entering the furnace? flow rate: 2.15 mol CH /min What is the molar flow rate of C,H,2 entering the furnace? 1.14 flow rate: mol C,H,2/min Incorrect What is the molar flow rate of the theoretical air required for the complete combustion of the fuel? 26.714 flow rate: mol air/min Incorrect What is the actual molar flow rate of air added to the furnace? 146.3 flow rate: mol air/min Incorrect What is the volumetric flow rate of air added to the furnace in standard cubic feet per minute? 166 flow rate: SCF/min Incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


