Question: Kindly help with question a and b (a) Distribution Law: if the solute species A are allowed to distribute itself between water and and organic
Kindly help with question a and b
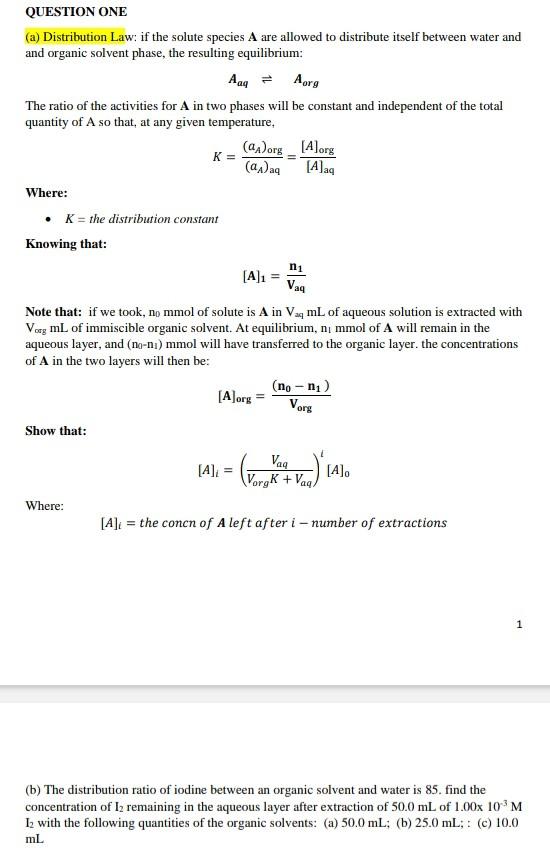
(a) Distribution Law: if the solute species A are allowed to distribute itself between water and and organic solvent phase, the resulting equilibrium: AaqAorg The ratio of the activities for A in two phases will be constant and independent of the total quantity of A so that, at any given temperature, K=(aA)aq(aA)org=[A]aq[A]org Where: - K= the distribution constant Knowing that: [A]1=Vaqn1 Note that: if we took, n0mmol of solute is A in VaqmL of aqueous solution is extracted with VorgmL of immiscible organic solvent. At equilibrium, n1mmol of A will remain in the aqueous layer, and (n0n1) mmol will have transferred to the organic layer. the concentrations of A in the two layers will then be: [A]org=Vorg(n0n1) Show that: [A]i=(VorgK+VaqVaq)i[A]0 Where: [A]i=theconcnofAleftafteri-numberofextractions 1 (b) The distribution ratio of iodine between an organic solvent and water is 85 . find the concentration of I2 remaining in the aqueous layer after extraction of 50.0mL of 1.00103M I 2 with the following quantities of the organic solvents: (a) 50.0mL (b) 25.0mL;: (c) 10.0 mL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


