Question: kindly help with this coursework. i tried to do it on my own firsf but i am failing to do calculation task from 4,5 and
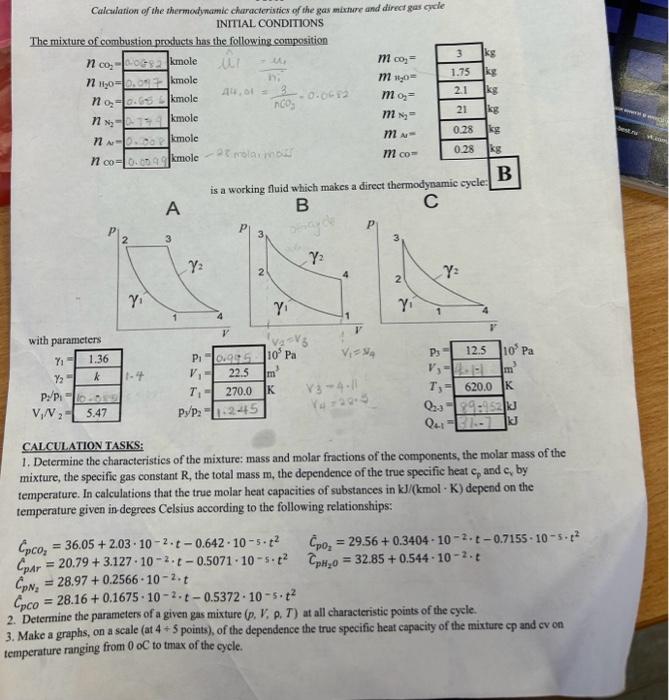
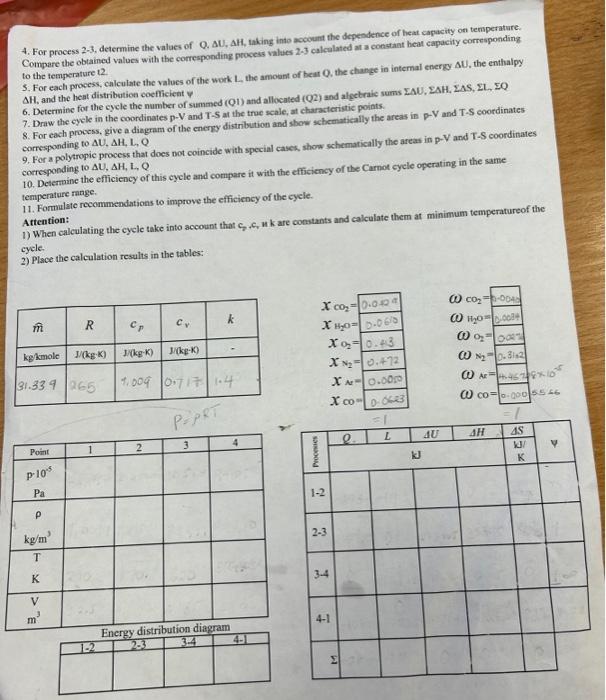




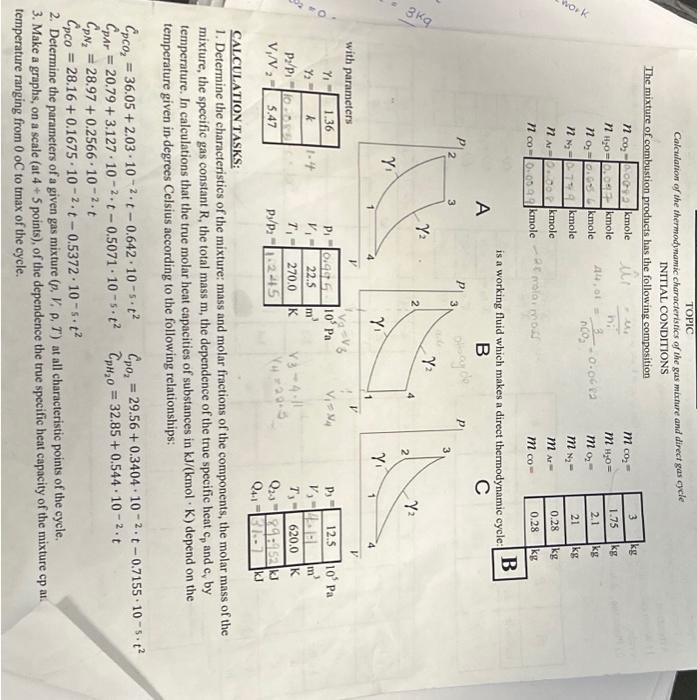
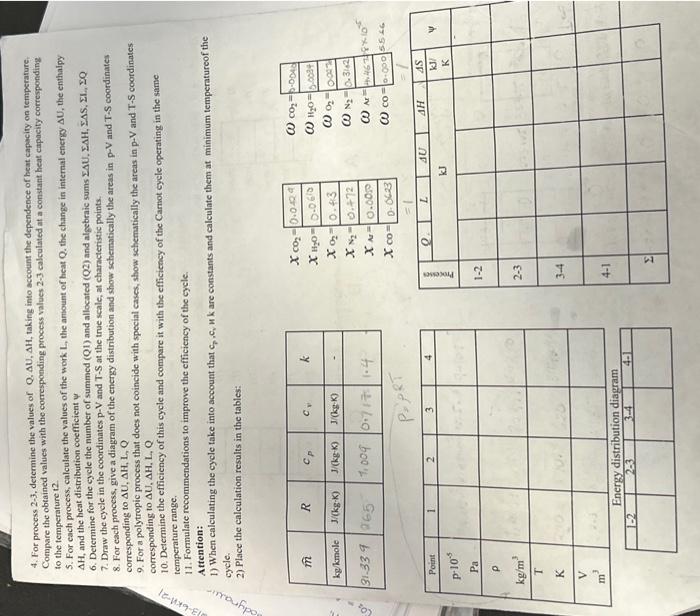
Calcularion of the thermody namic characteristics of the gas mixmare and direct gas cycle INITAL CONDITIONS The mixture of combustion products has the following compesition is a working fluid which makes a direct thermodynamic cycle: D CALCULATION TASKS: 1. Determine the characteristics of the mixture: mass and molar fractions of the components, the molar mass of the mixture, the specific gas constant R, the total mass m, the dependence of the true specifie heat cp and cs by temperature. In calculations that the true molar heat capacities of substances in kJ/(kmolK) depend on the temperature given in-degrees Celsius according to the following relationships: CpCO2=36.05+2.03102t0.642105t2Cp01=29.56+0.340410=2t0.7155105t2CpArA=20.79+3.127102t0.5071105t2CpH20=32.85+0.544102.tCpN2=28.97+0.2566102tCpCO=28.16+0.1675102t0.5372105t2 2. Detcrmine the parameters of a given gas mixture (p,V,P,T) at all characteristic points of the cycle. 3. Make a graphs, on a seale (at 4+5 points), of the dependence the true specific heat capacity of the mixture cp and cv on emperafure ranging from 0 oC to tmax of the cycle. 4. For process 23, determine the values of Q. U, AH, taking into acoount the dependence of heat capacity on tersperature. Compare the obtained values with the corresponding process values 23 calcalated at a coestant beat capacity corresponding to the temperature t2. 5. For each proecss, calculate the values of the work L. the amount of beat Q. the change in intemal energy U, the enthalpy AH, and the hest distribution coeflicient of 7. Draw the eycle in the coordinates pV and T5 at the true scale, at characteristic points. 8. For cach process, give a diagram of the enerty distribution and show schematically the areas in pV and TS coordinates corresponding to AU,AH,L,Q 9. For a polytropic process that does not coincide with special cases, show schematically the areas in pV and TS coordinates corresponding to AL,H,L,Q 10. Detertaine the efficiency of this cycle and compare it with the efficiency of the Carnot cycle operating in the same temperature tange. 11. Formulate recommendations to improve the efficiency of the cycle. Aftention: Atication: 1) When calculating the cycle take into account that c,,c,,kk are contants and calculate them at minimum temperatureof the cycle. 2) Place the calculation results in the tables: ^i=n1i^CO2=44,01kg/ole44,07kglucle=nCO3kgnCO2=0.0682kuole Gharactenehes of mixtide i+iKni=4.588yKuole Siculate uole nackian xCO2=nunxnCO2=10rS10.0682=0.0429xH2O=nM10nH20=70Rri0.097=0.0610x2=nixn02=1.5B81c.05t=0.413x=N2=narixnn0=1.0rri0.141=0.472xA,=nWirnar=1.08810.008=0.0000xco=1.58910.099=0.0.623 Ealiculate uolai ua-es of g A = uixt.n =(0.052325.01)=31.339kgwol Calculate raes fruction of couponente 000Atereu60x40=31+3390.350.0623=0.000.50ts iilculote specific goo mrotamt R=A^R^=31339844=0265 tculate fotal Nasit ulixlue: (3+0.0.45.2)+(1.150.097)+(2,10.65x)+(210.049)+((0.28y.0.0094)=17.4859 wilutate apecific heot 2. Parqucters of given gas suixtrie: (12) Adiabat c campicgerion: pv=CP1v1x=P2V2P1P2=(v1v2)1T1T2=(V0v1)(111) x1=1362=5A72=92.5m31=9.0KT1=970 500T2T2=T1(V4V1)8=210(5.47)1361497782k P1Pe=(v2v1)x1=54713P1P2=10.089v0=5.47v15.4422.5+4.113 Consideiect theconstant volume proceiss (23) TP=C frou given T3=620K P8P3T4T3=497.782620P3P3=1.245 P3=12.5105PaP3=12.5105P9P2=1240P3=1.94512.5105=10.04105PgPn=100152=100151000+50=0,495106Pq as it 15 ionstant volyaie he qt aidestan phocod V2=V2=411 is For adiabatic expansion process (8) (P4P3)=197201P3=1.970414=10.73PH=1073PB=10.73125105=1165105Pq during protess (2-3) theie is heat supplied at constant voluue per unit hase (u1kg)Q23=C1(T3T2)C=11R+13610.265=0.736KgKKQ23=0.736(620497782)=rg9.52kJ Simiariy During CA1) we have heat rejection process Q41=Cv(T4T1)C1=K1R=1410.287=0.717KgKKJQ41=0.717(314.2270)=31.7KJ TOPIC Calcularion of the thermodynamic characterisnics of the gas mture and direct gas gele INIIIAL CONDITIONS The mixture of combustion products has the following composition is a working fluid which makes a direct thermodynamic cycle: D CALCULATIONTASKS: 1. Determine the characteristics of the mixture: mass and molar fractions of the components, the molar mass of the mixture, the specific gas constant R, the total mass m, the dependence of the true specific heat cp and cy by temperature. In calculations that the true molar heat capacities of substances in kJ/(kmolK) depend on the temperature given in degrees Celsius according to the following relationships: CpCO2=36.05+2.03102t0.642105t2CDAr=20.79+3.127102t0.5071105t2CpN2=28.97+0.2566102tCpCO=28.16+0.1675102t0.5372105t2CpO2=29.56+0.3404102t0.7155105t2CpH2O=32.85+0.544102t 2. Determine the parameters of a given gas mixture (p,V,,T) at all characteristic points of the cycle. 3. Make a graphs, on a scale (at 4+5 points), of the dependence the true specific heat capacity of the mixture cp at emperature ranging from 0oC to tmax of the cycle. 4. For process 2-3, determine the values of Q.U,H, taking into account the dependeoce of heat capacity on temperafure. Compare the obtained values with the corresponding process values 23 calculated at a constant heat capacity corresponding to the temperature t? 5. For cach process, calculate the values of the work L. the amount of heat Q. the change in internal enerigy U, the enthalpy. AH, and the heat distribution coefticient 7. Draw the eycle in the coordinates PV and TS at the true scale, at characteristic points. 8. For cach process, give a diagram of the encrgy distribution and show schematically the areas in pV and T-S coordinates corresponding to U,AH, I, Q 9. For a polytropic process that does not coincide with special cases, shory sehematically the areas in pV and T-S coordinates. corresponding to U,H,L,Q 10. Determine the efficiency of this cycle and consare it with the efficiency of the Carmot cycle operating in the samse. temperature range. 11. Formulate recommendations to improve the efticiency of the eycle. Attention: 1) When calculating the cycle take into account that c,,c,K are constants and calculate them at minimum temperaturcof the cycle. 2) Place the calculation results in the tables
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


