Question: kinetic iodine clock reaction Report Table C 3. Initial Concentrations Table view List view Concentrations of reagents 1,0,21. (M) 1'1(M) Brolo (M) TH (M) 0.001
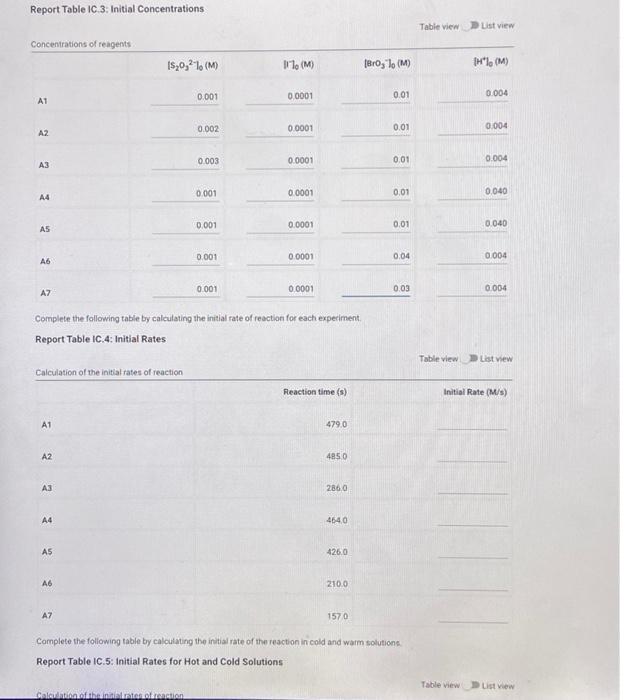
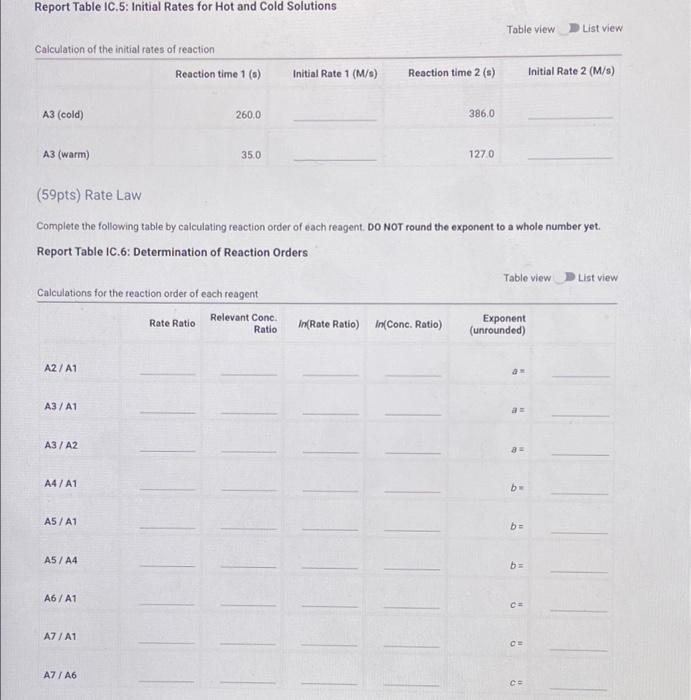
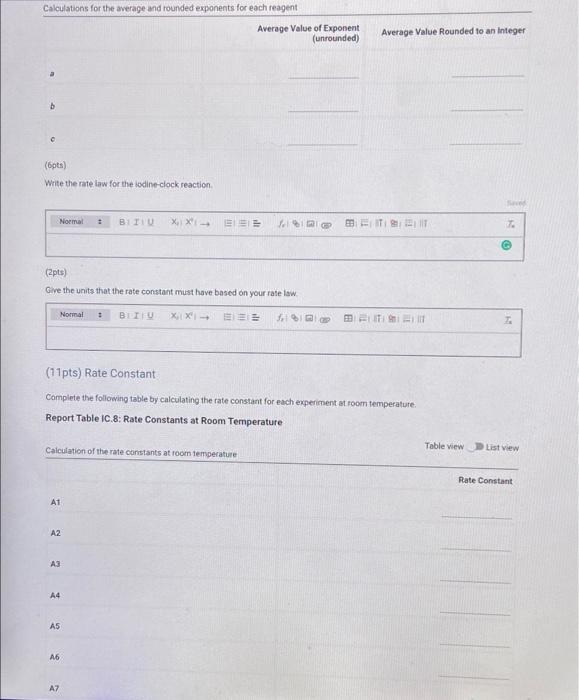

Report Table C 3. Initial Concentrations Table view List view Concentrations of reagents 1,0,21. (M) 1'1(M) Brolo (M) TH" (M) 0.001 0.0001 0.01 0.004 A1 0.002 AZ 0.0001 0.01 0.004 0.003 A3 0.0001 0.01 0.004 A4 0.001 0.0001 0.01 0.040 0.001 A5 0.0001 0.01 0.040 0.001 0.0001 0.04 A6 0.004 0.001 0.0001 0.03 AZ 0.004 Complete the following table by calculating the initial rate of reaction for each experiment, Report Table IC.4: Initial Rates Table view List view Calculation of the initial rates of reaction Reaction time (s) Initial Rate (M/s) A1 479,0 A2 4850 A3 2860 A4 4640 A5 4260 A6 210.0 A7 1570 Complete the following table by calculating the initial rate of the reaction in cold and warm solutions Report Table IC.5: Initial Rates for Hot and Cold Solutions Table view List view Solatban Report Table IC.5: Initial Rates for Hot and Cold Solutions Table view List view Calculation of the initial rates of reaction Reaction time 1) Initial Rate 1 (M/8) Reaction time 2 (s) Initial Rate 2 (M/S) A3 (cold) 260.0 386,0 A3 (warm) 35.0 1270 (59pts) Rate Law Complete the following table by calculating reaction order of each reagent. DO NOT round the exponent to a whole number yet. Report Table IC.6: Determination of Reaction Orders Table view Calculations for the reaction order of each reagent In(Rate Ratio) In(Conc. Ratio) Exponent (unrounded) List View Rate Ratio Relevant Cone. Ratio A2/A1 A3/A1 A3/A2 a A4/A1 b A5/A1 b= A5 / A4 b= A6/A1 A7/A1 CE A7 / A6 CE Calculations for the average and rounded exponents for each reagent Average Value of Exponent (unrounded) Average Value Rounded to an integer c (opts) Write the rate law for the lodine-clock reaction Normal + BIRU X, X ESE BEIT (2pts) Give the units that the rate constant must have based on your rate law Normal 1 BIO $1$ I (11 pts) Rate Constant Complete the following table by calculating the rate constant for each experimentat room temperature Report Table 10.8: Rate Constants at Room Temperature Calculation of the rate constants at room temperature Table view List View Rate Constant 1 A2 A3 A4 A5 A6 A7 Report Table IC.9: Rate Constants at Warm and Cold Temperatures Table view List view Calculation of the rate constants at different temperatures Rate Constant 1 Rate Constant 2 A3 (cold) A3 (warm) (5pts) Determination of the Activation Energy Be sure to report values with the correct number of significant figures. (1pts) Average value of rate constant: (exclude A3 cold and A3 warm values) (1pts) Average rate constant in cold water: k- (1pts) Average rate constant in warm water k= Temperature of reagents at room temperature: Temperature of ice water bath: 220C Temperature of warm water bath 35.0C 50.0 C (1pts) Slope from Arrhenius plot m= (1pts) Activation Energy. Es
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


