Question: Kinetics 1 Homework Due Friday, 1 - 1 2 - 2 4 Show your work to receive full credit. You can write your answers on
Kinetics Homework
Due Friday,
Show your work to receive full credit. You can write your answers on this paper or use a
separate sheet of paper.
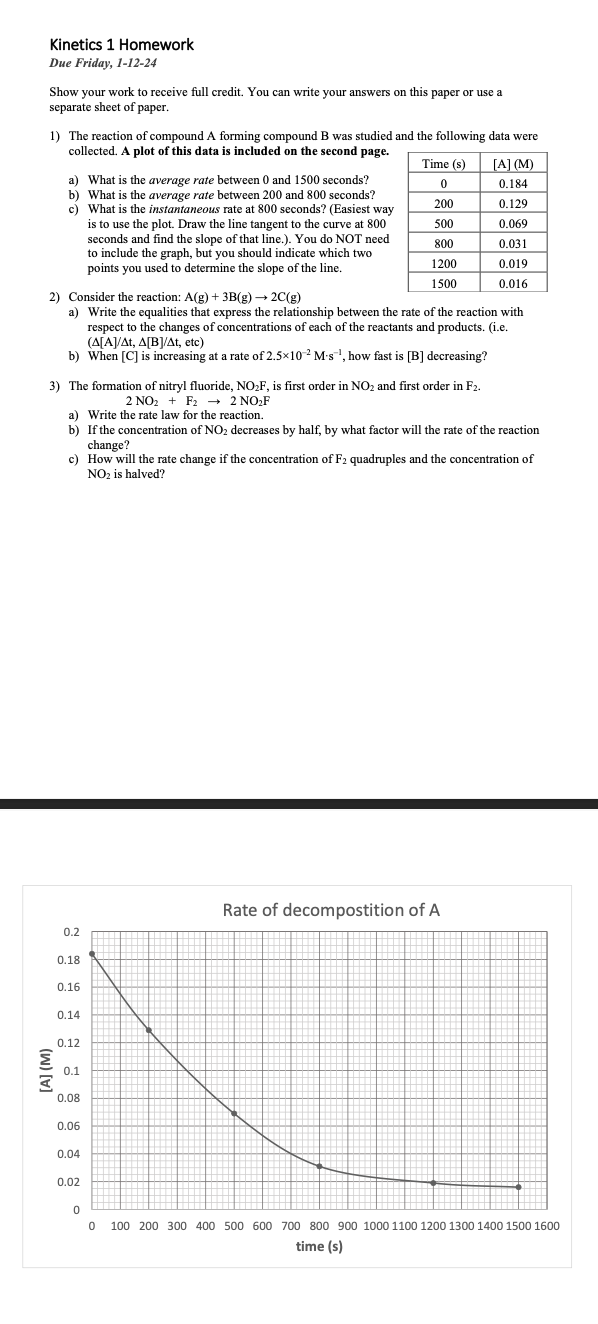
The reaction of compound A forming compound was studied and the following data were
collected. A plot of this data is included on the second page.
a What is the average rate between and seconds?
b What is the average rate between and seconds?
c What is the instantaneous rate at seconds? Easiest way
is to use the plot. Draw the line tangent to the curve at
seconds and find the slope of that line. You do NOT need
to include the graph, but you should indicate which two
points you used to determine the slope of the line.
Consider the reaction:
a Write the equalities that express the relationship between the rate of the reaction with
respect to the changes of concentrations of each of the reactants and products. ie
etc
b When is increasing at a rate of how fast is decreasing?
The formation of nitryl fluoride, is first order in and first order in
a Write the rate law for the reaction.
b If the concentration of decreases by half, by what factor will the rate of the reaction
change?
c How will the rate change if the concentration of quadruples and the concentration of
is halved?Rate of decompostition of AKinetics Homework
Due Friday,
Show your work to receive full credit. You can write your answers on this paper or use a
separate sheet of paper.
The reaction of compound A forming compound was studied and the following data were
collected. A plot of this data is included on the second page.
a What is the average rate between and seconds?
b What is the average rate between and seconds?
c What is the instantaneous rate at seconds? Easiest way
is to use the plot. Draw the line tangent to the curve at
seconds and find the slope of that line. You do NOT need
to include the graph, but you should indicate which two
points you used to determine the slope of the line.
Consider the reaction:
a Write the equalities that express the relationship between the rate of the reaction with
respect to the changes of concentrations of each of the reactants and products. ie
etc
b When is increasing at a rate of how fast is B decreasing?
The formation of nitryl fluoride, is first order in and first order in
a Write the rate law for the reaction.
b If the concentration of decreases by half, by what factor will the rate of the reaction
change?
c How will the rate change if the concentration of quadruples and the concentration of
is halved?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


