Question: Kinetics problem: = Give the final equation that can be used to solve for the equilibrium conversion for each case given: k= known value, V(Reactor/Reaction

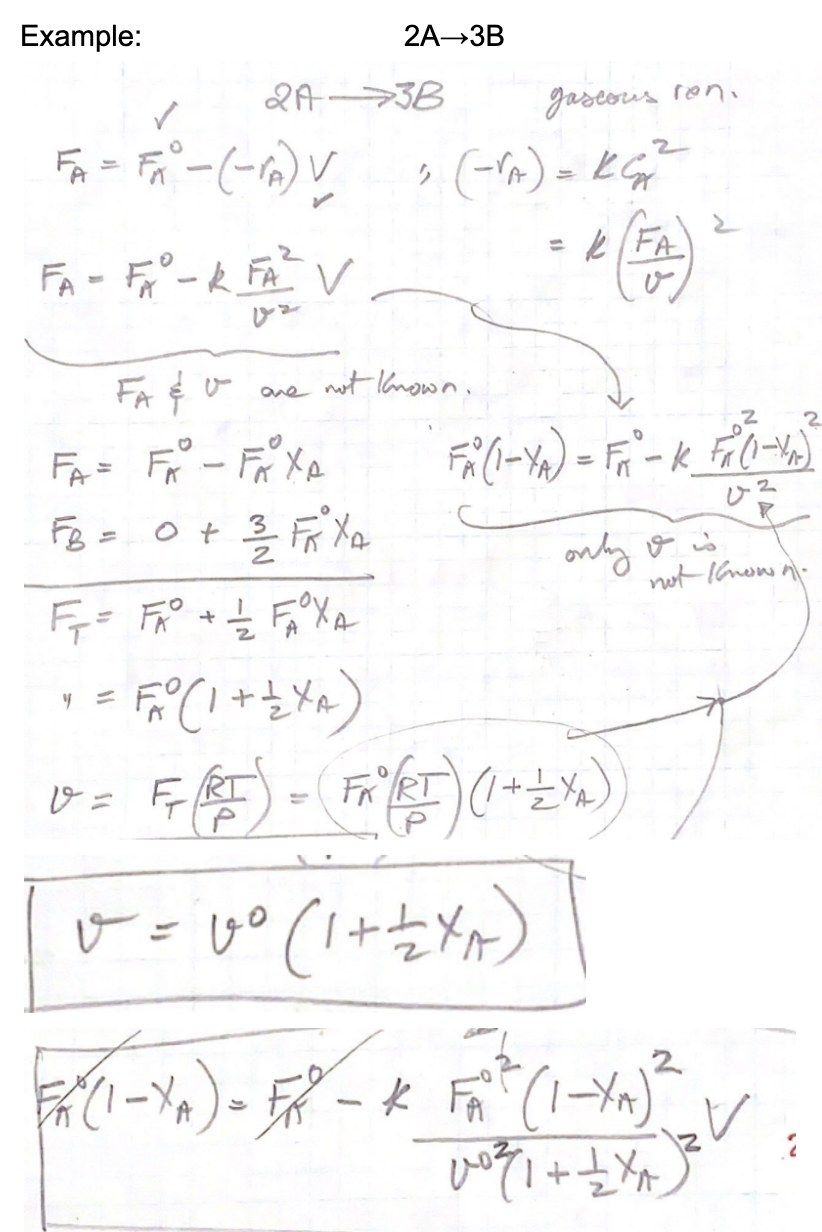
Kinetics problem: = Give the final equation that can be used to solve for the equilibrium conversion for each case given: k= known value, V(Reactor/Reaction Volume in liters)=known value ,vo (liter/min) = known value, FAo (mole/min) = known value, isothermal gaseous rxn Constant Volume reactor: a) AB b) 2A_B c) 3AB d) A+B-C e) A-2B+C f) A+B-C g) 2A+B-C h) A2B Example: 2A3B 24->3B ascous reni 2 FA = FM - (-AV (-A) = RG RFA FA - FA - K FA v v 2 : 2 only & in not known 2 y = are not known. FA= FA - FAXA FX (1-YA) = Friuk Fall FB = 0 + 3 Foto = FR + 2 FOXA FA (1 + 24A) DE FIE) - (+2) v= RI ) % v=vo (1+2+A) x FF - K F (1-7A)? V * 0031 + 12 YA FA/RT 2 1-Yo)-58-4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


