Question: L B P Two containers (A and B) are connected using a pipe (length:L=20cm, diameter: D). Both A and B are filled with stagnant acetic
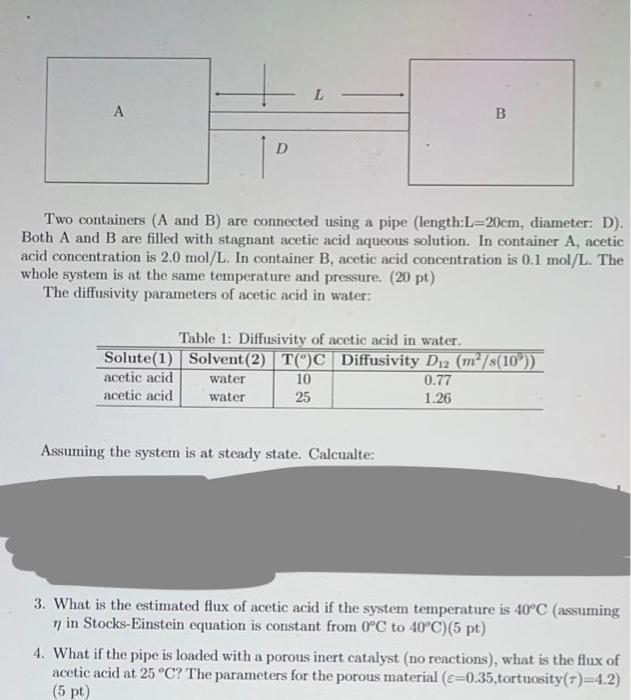
L B P Two containers (A and B) are connected using a pipe (length:L=20cm, diameter: D). Both A and B are filled with stagnant acetic acid aqueous solution. In container A, acetic acid concentration is 2.0 mol/L. In container B, acetic acid concentration is 0.1 mol/L. The whole system is at the same temperature and pressure. (20 pt) The diffusivity parameters of acetic acid in water: Table 1: Diffusivity of acetic acid in water. Solute(1) Solvent(2) TOC Diffusivity Di2 (m/s(10%)) acetic acid water 10 0.77 acetic acid water 25 1.26 Assuming the system is at steady state. Calcualte: 3. What is the estimated flux of acetic acid if the system temperature is 40C (assuming n in Stocks-Einstein equation is constant from 0C to 40C)(5 pt) 4. What if the pipe is loaded with a porous inert catalyst (no reactions), what is the flux of acetic acid at 25C? The parameters for the porous material (c=0.35, tortuosity(t)=4.2) (5 pt)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


