Question: Lab #4-Flame Test Data Table Part A - Simple Flame Test Part B: Line Spectra (questions will be asked on the Lab Report on Canvas)
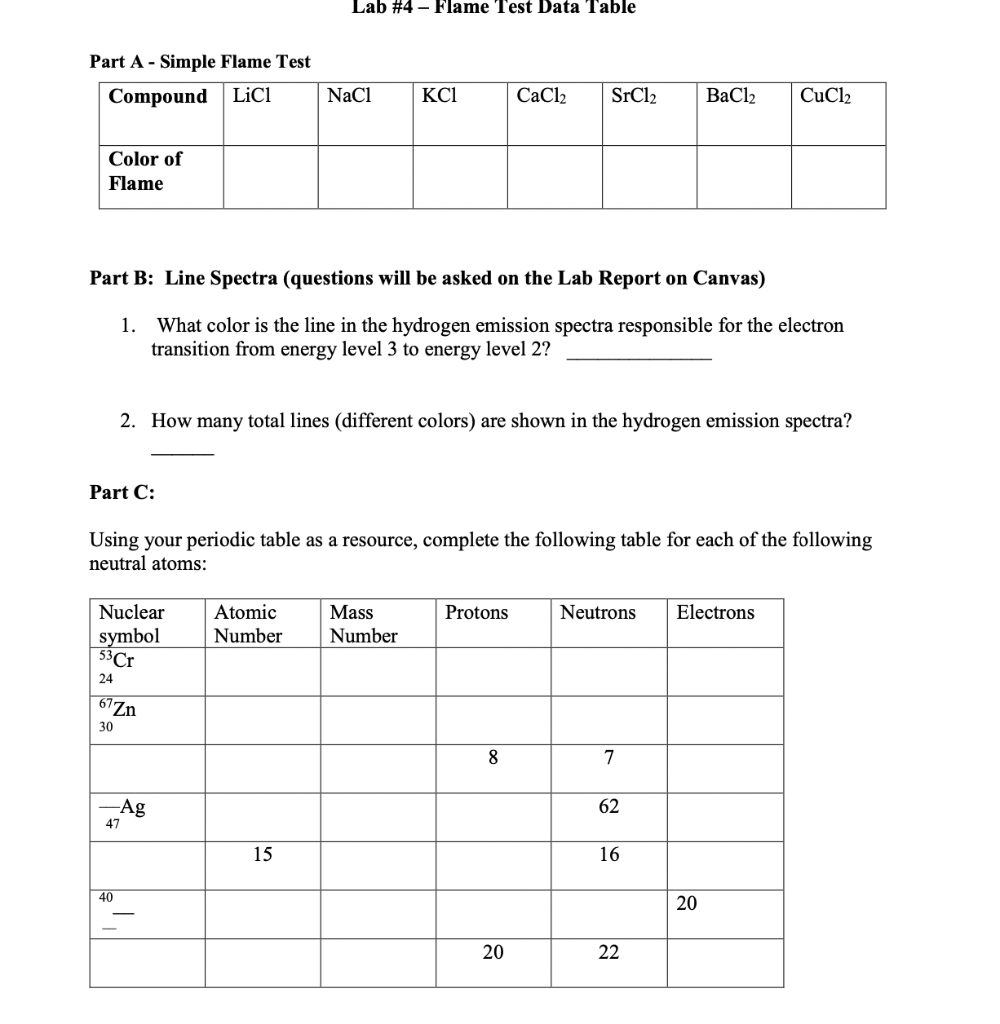
Lab \#4-Flame Test Data Table Part A - Simple Flame Test Part B: Line Spectra (questions will be asked on the Lab Report on Canvas) 1. What color is the line in the hydrogen emission spectra responsible for the electron transition from energy level 3 to energy level 2 ? 2. How many total lines (different colors) are shown in the hydrogen emission spectra? Part C: Using your periodic table as a resource, complete the following table for each of the following neutral atoms
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


