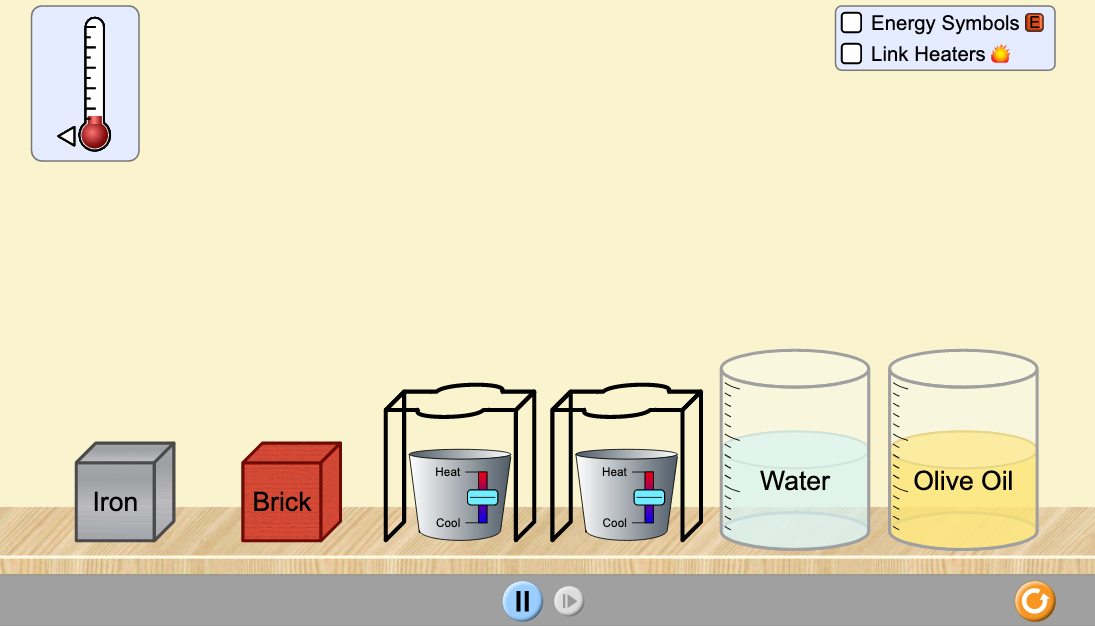
Question: Lab 9A. Energy Forms & Changes Name _____________________ https://phet.colorado.edu/sims/html/energy-forms-and-changes/latest/energy-forms-and-changes_en.html?stickyBurners Lab 9A Goal: Intro to Energy : what is it? What does it do? How do
Lab 9A. Energy Forms & Changes Name _____________________
https://phet.colorado.edu/sims/html/energy-forms-and-changes/latest/energy-forms-and-changes_en.html?stickyBurners
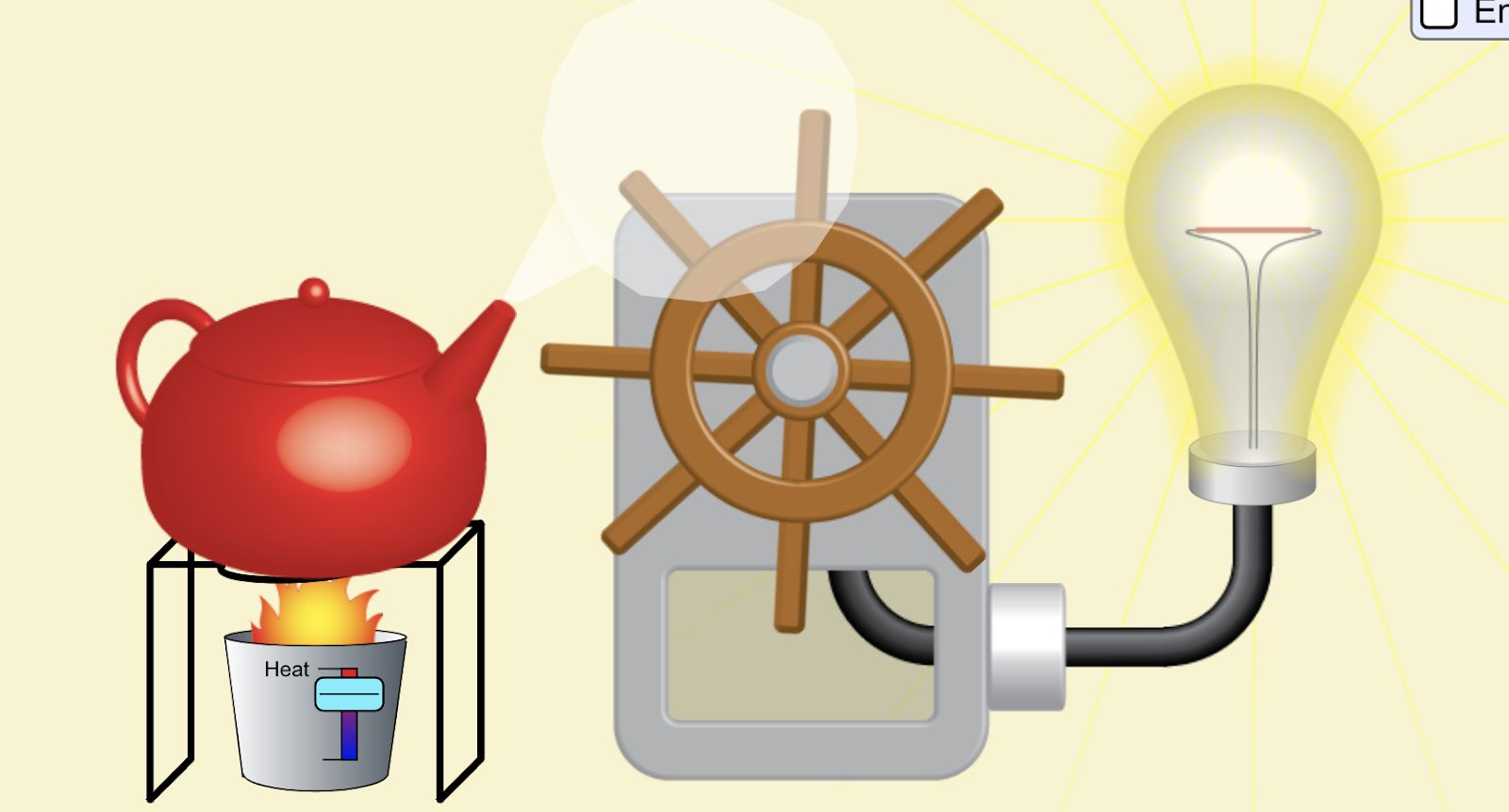
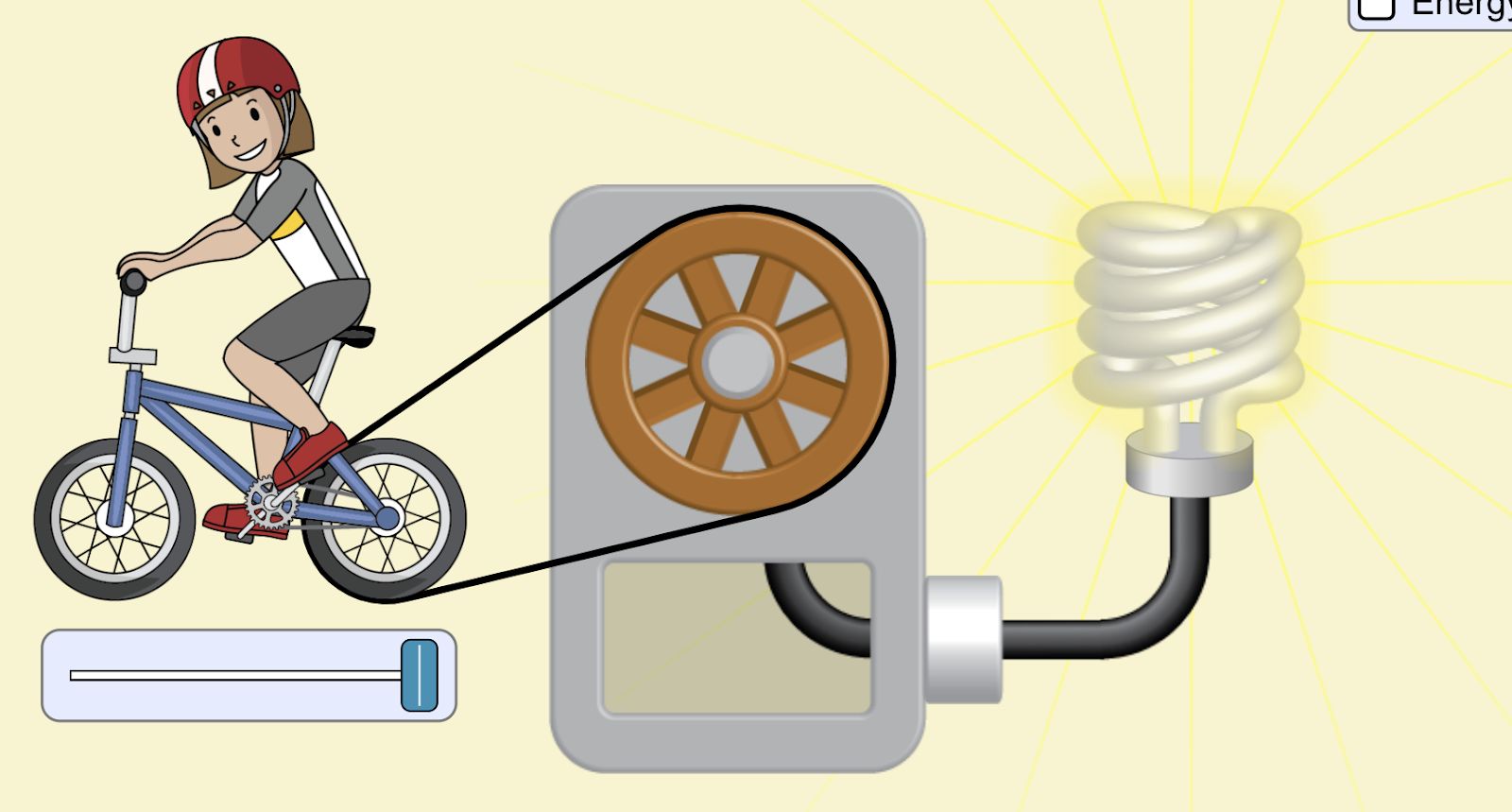
Lab 9A Goal: Intro to Energy: what is it? What does it do? How do we characterize it? How do we measure it?
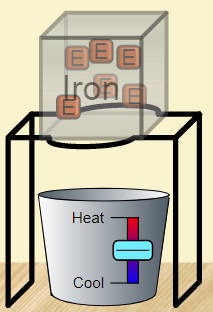
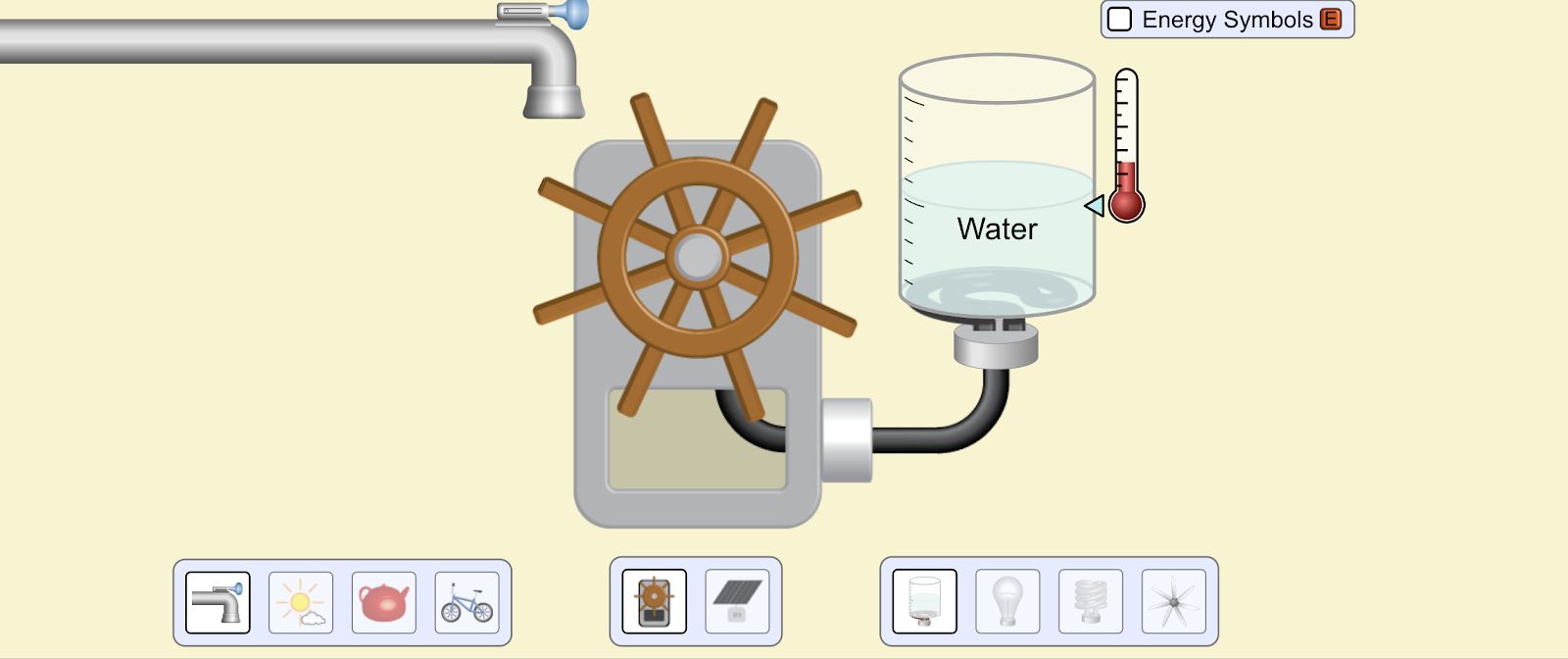

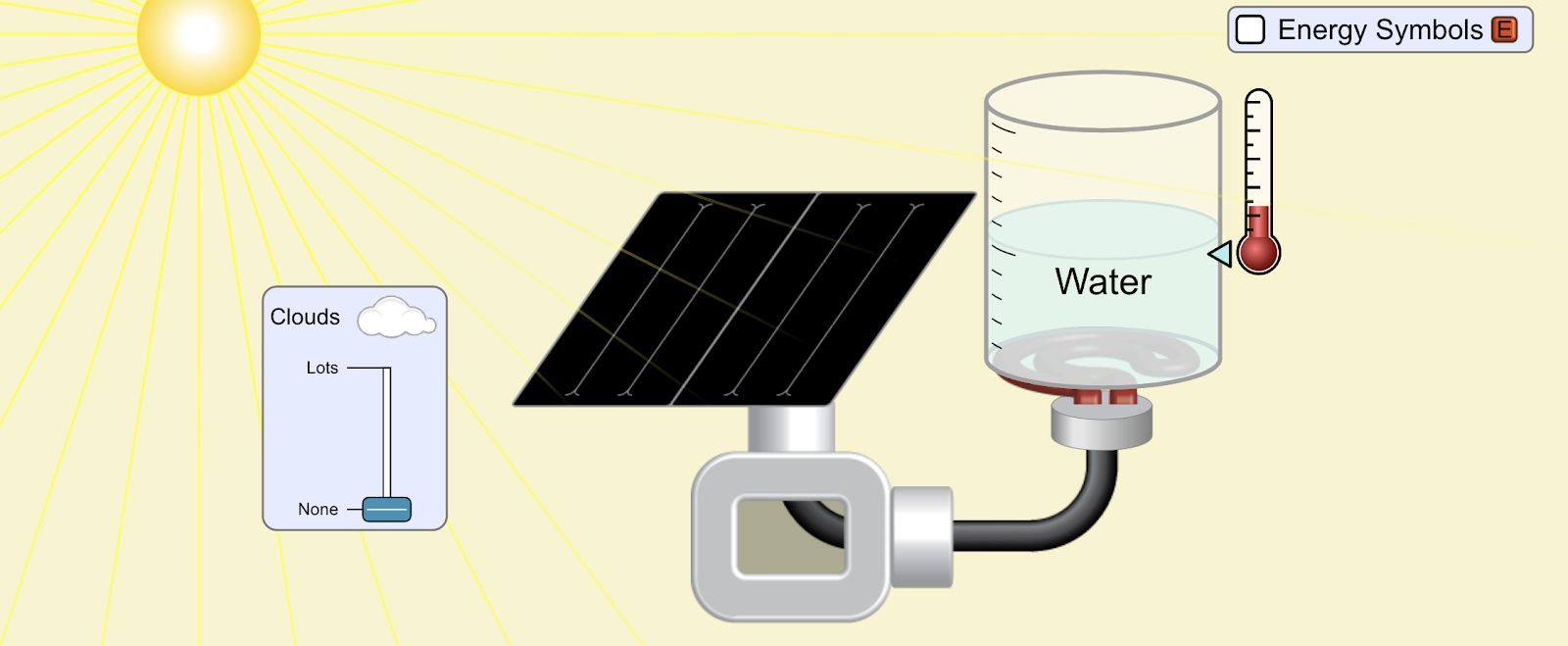

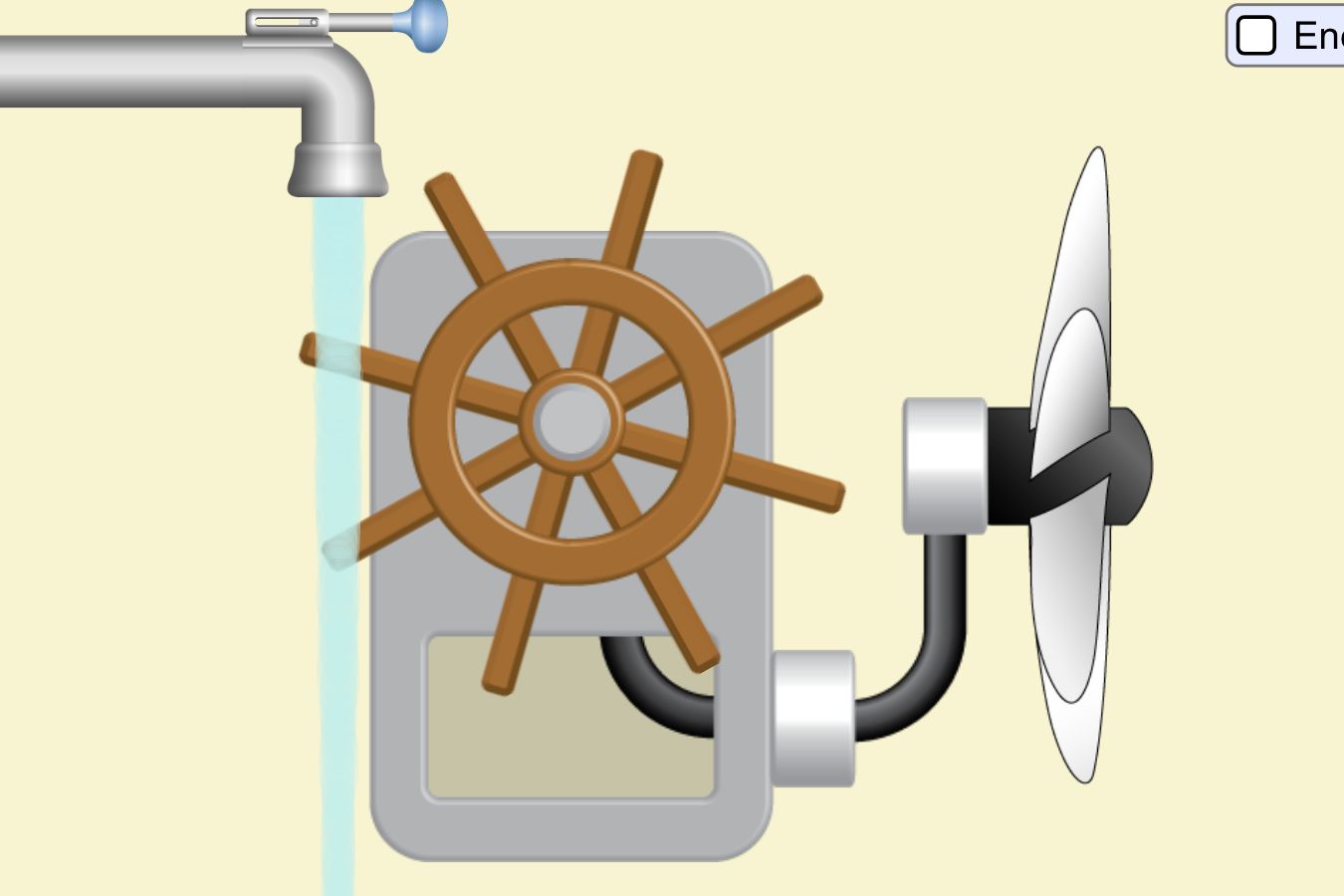

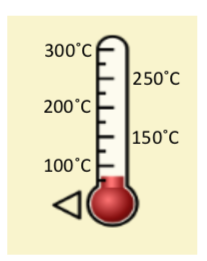
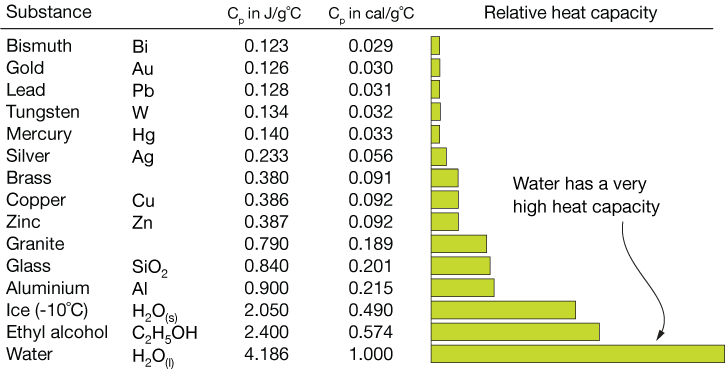
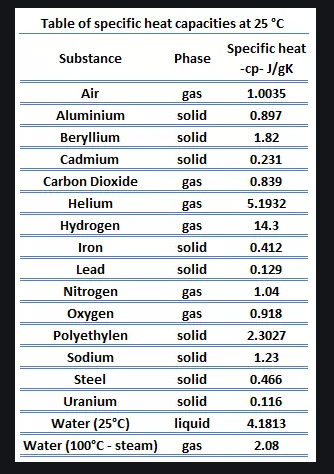
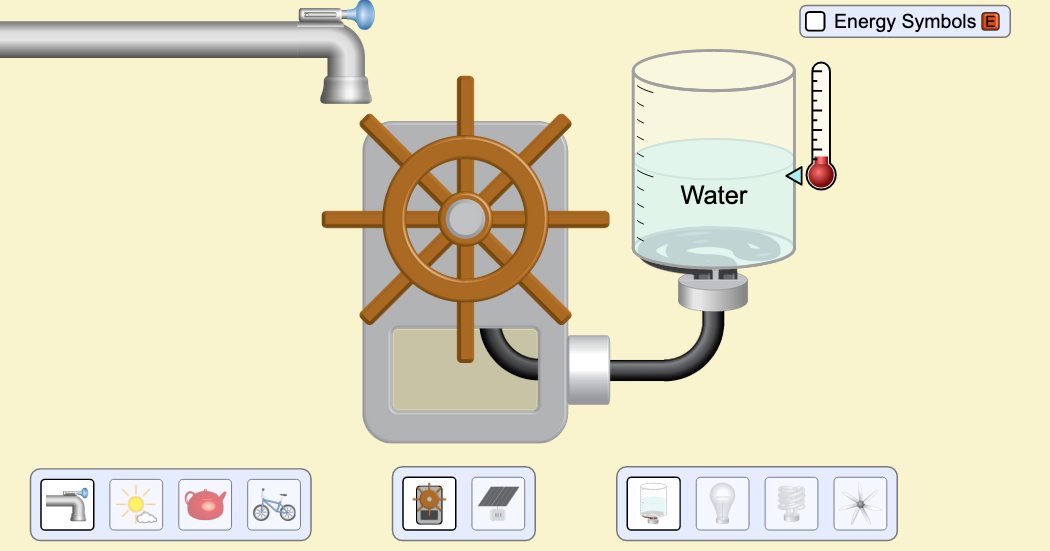
\f300 C TT 250 C 200 C 150 C 100 *CSubeisrios in exl/ G Relative heat capacity Btermmath 0. 123 Gold All Pb 0. 123 0.031 Tunigetari 0. 134 0.032 Mercury Hig 0. 140 Silver 0.05S Brass 0 380 0.091 Water has a very Copper high heat capacity Zine 0.38 0.082 Granite 0.790 0.840 0.201 Aluminium Al 0900 4 215 2.050 0.490 Ethyl alcohol CHOH 2.400 0.574 HOT 4.185 1.000Table of specific heat capacities at 25 "C Substance Specific heat Phase -cp- J/gK Air gas 1.0035 Aluminium solid 0.897 Beryllium solid 1.82 Cadmium solid 0.231 Carbon Dioxide gas 0.839 Helium gas 5.1932 Hydrogen gas 14.3 Iron solid 0.412 Lead solid 0.129 Nitrogen gas 1.04 Oxygen gas 0.918 Polyethylen solid 2.3027 Sodium solid 1.23 Steel solid 0.466 Uranium solid 0.116 Water (25*c) liquid 4.1813 Water (100"C - steam) gas 2.08
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


