Question: Lab Report #12 - Voltaic Cells Measured cell potentials Edll (measured) 1,086 1.053 1.121 0.626 0.618 0.643 1.504 1. ZnZn?(1.0M) Cu? (1.0M) Cu 2. ZnZn?(1.0M)
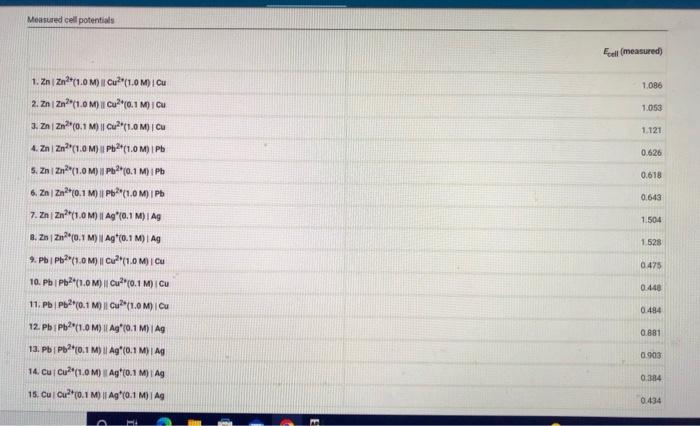
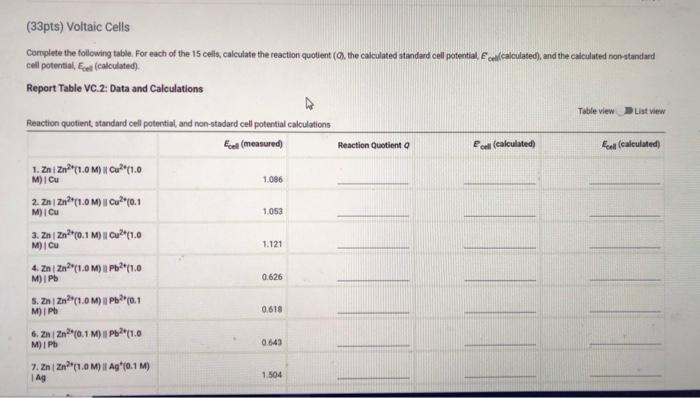
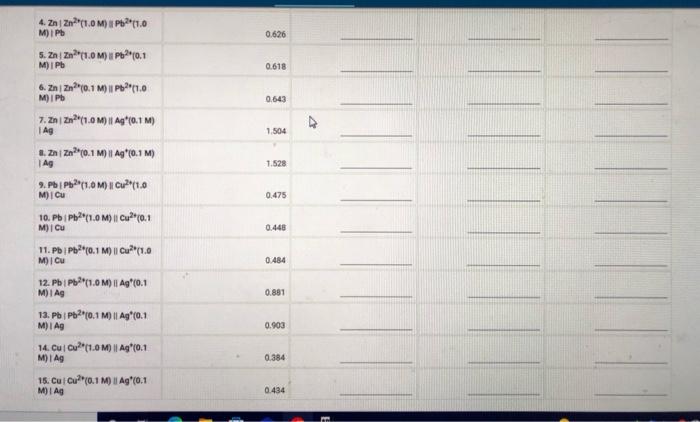
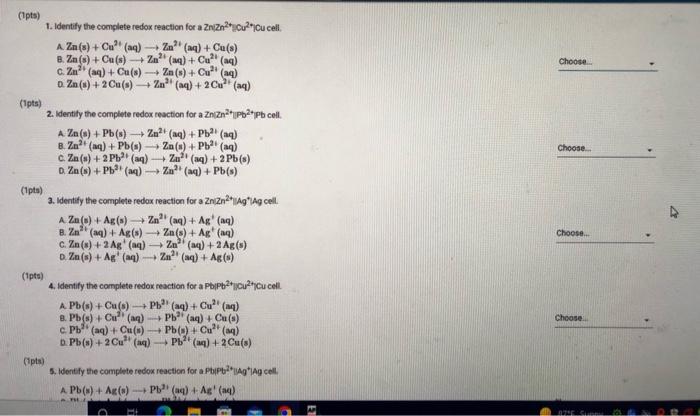
Measured cell potentials Edll (measured) 1,086 1.053 1.121 0.626 0.618 0.643 1.504 1. ZnZn?"(1.0M) Cu? (1.0M) Cu 2. ZnZn?"(1.0M) Cu (0.1 MOI CU 3. ZnZn0.1 M) || (1.0M) CU 4. Zn/Zn?"(1.0M) 11Pb2(1.0M) I Pb S. Zni Zn2+(1.0M) || Pb2+0.1 M) i Pb 6. ZnZn (0.1 M) | P(1.0M) Pb 7. ZnZn?"(1.0M) || Aq"(0,1 M)| Ag 8.2 | Zn(0.1 M) | Ag*(0.1 M) | AG 9. PbPb?"(1.0M) || Cu?"(1.0M) | CU 10. PbPb (1.0M) || Cu?"(0.1 M) CU 11. PbPb(0.1 M) | Cu*(1.0M) ICU 12. Pb Pb?"(1.0M) | Ag (0,1 M) TAG 13. PbPb20.1 M) | Ag*(0,1 M) TAG 14. Cui Cu?" (1.0M) Ag (0.1 M) | AG 15. Cui Cu?(0.1 M) || Ag (0.1 M) 1.528 0.478 0.440 0.484 0.881 0.903 0.384 0.434 (33pts) Voltaic Cells Complete the following table. For each of the 15 cells, calculate the reaction quotient (9. the calculated standard cell potential. Podcalculated), and the calculated non-standard cell potential, Ece (calculated) Report Table VC.2: Data and Calculations Table view List View Reaction Quotient a cell (calculated) Ecell (calculated 1.086 Reaction quotient standard cell potential, and non-stadard cell potential calculations Esel (measured 1. Zn Zn"1.0M) | Cu?"(1.0 MICU 2. Zni zn*(1.0M) || Cu? (0.1 Mi Cu 3. Zn | Zn(0.1 M) | Cu2+(1.0 M) Cu 4. ZnZn(1.0M) Pb(1.0 M) IPB S. ZnZn (1.0M) Pb(0.1 1.053 1.121 0.626 M) iPh 0.618 6.22(0.1 M) Pb(1.0 M) IPO 0.643 7. ZnZn?"(1.0M) || Ag*0.1 M) 1 AG 1.504 0.626 0.618 0.643 V 1.504 1.528 0.475 4. ZnZn?"(1.0MP2t1.0 M) Pb 5. ZnZn"(1.0M) UPPH(0,1 M) I Pb 6. ZnZn10.1 M) | Pb 11.0 M) I Pb 7. ZnZn(1.0M) 11 Ag*(0,1M) Ag 1. ZnZn?" (0.1 M) 1 Ag"(0.1M Ag 2.PbPb?" (1.0M) 1 Cu2(1.0 M) I Cu 10. Pb/Pb2(1.0 M) || Cu*(0,1 M) Cu 11. Pb | Pb?*(0.1 M) || Cu?*(1.0 M) Cu 12. Pb Pb (1.0M) 11 Ag (0.1 M) IAS 13. PbPb(0,1 M) || Ag* (0.1 M) Ag 14. Cucu (1.0M) || Ag (0.1 M) Ag 16. Cui Cu? (0.1 M) | Ag (0.1 M) Ag 0.448 0.484 0881 0.903 0.384 0.434 Choose Z? Choose.. (Ipts) 1. Identify the complete redox reaction for a Zniznucu2"Cu cell A Zn(s) + Cu? (aq) Zn (4) + Ca(s) 2. Zn(s) + Cu(s) + Zu? (aq) + Cu?! (4) C. Zu (aq) + Cu(8) + Zn (8) + Cu? (aq) o. Zn(s) + 2Cu() (aq) + 2C (2) (1pts) 2. Identify the complete redox reaction for a Znizauppapb cell. A Zn(s+ Pb(s) + Zn? (aq) + Pb (aq) B. Zm2 (na) + Pb() 2(8) + Pb2+ (aq) c. Za(s) + 2 Pb (4) Zal(aq) + 2 Pb(s) D. Ze (8) + Pb(aq) Zn (aq) + Pb(s) (tts) 3. Identify the complete redox reaction for a ZnZnAgAg cell. A Za(s) + Ag() Zn(aq) + Ag+ (aq) a. Zn(4) + Ag(s) - Z) + Ag (2) c.Zn(s) + 2 Ag (4) 2 (aq) + 2 Ag(s) D. Zn(s) + Ag (4) Za (14) + Ag(s) (pt) 4. Identify the complete redox reaction for a Pb/Pbc2jcu cell. A Pb(s) + Cu(s) - P(q) + Cu? (14) 8. Pb(s) + Cu? (aq) + (aq) Cu(s) c Pb (aq) + Cu(n) Pb( ) Cu? (14) Pb(s) + 2 Cu? (aq) Pb (14) + Cu(s) Cipts) 5. Identify the complete redox reaction for a (Pb2*g*lag cell A Pb(s) + Ac(s) PV (4) Ag (4) Choose.. Choose BE REPORT SUMMARY CPS () Car P5C () D. Pb(s) + 2Cu? (4) Pb () + 2 Cu(s) (Ipts) 5. Identify the complete redox reaction for a Pipog'Ag cell A Pb(s) + Ag(s) PPP (aq) + Ag" (aq) B. Pb(s) + Ag (4) P2 (aq) + Ag(s) c. Pb2(aq) + Ag(s) Pb(8) + Ag" (aq) o. Pb(s) + 2 Ag" (n) Pb (14) +2 Ag(s) Choose... (pts) 6. Identify the complete redox reaction for a Cucu?"Ag*Ag cell A Cu(s) + Ag(s) Cu (aq) + Ag" () B. Cu (s) + Ag" (n) Cu (aq) + Ag(s) c Cu(s) + 2 Ag' (a) Cu? (aq) + 2 Ag(s) a. Cu (aq) + 2 Ag(s) Cu(s) + 2 Ag+ (aq) Choose (1pts) Calculation Uploads (tts) Upload images of your work for one sample calculation each of reaction quotient, Ecel (calculated) and Ece (calc), including units. Browse your files to upload or Drag and Drop Max attachments: 51 Mex Size 20.00MB each
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


