Question: Lead(II) oxide can be reduced to form elemental lead through the reaction: PbO(s)+C(s)Pb(s)+CO(g) Calculate H for the above reaction given the following (all values are
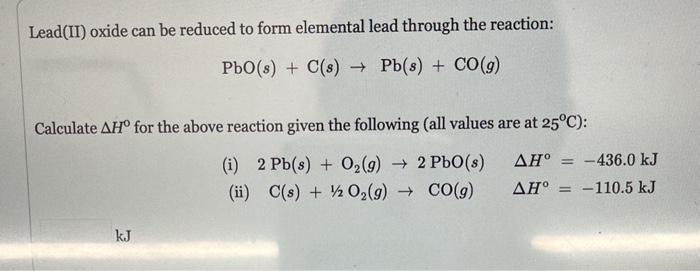
Lead(II) oxide can be reduced to form elemental lead through the reaction: PbO(s)+C(s)Pb(s)+CO(g) Calculate H for the above reaction given the following (all values are at 25C ): (i) 2Pb(s)+O2(g)2PbO(s)H=436.0kJ (ii) C(s)+1/2O2(g)CO(g)H=110.5kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


