Question: LEASE HELP ASAP. PLEASE SOLVE IT CORRECTLY, DONT COPY OTHER SOLUTIONS. SOLVE ALL PARTS. WRITE DOWN ALL OF YOUR ASSUMPTIONS. Remember that Mixture can be
LEASE HELP ASAP. PLEASE SOLVE IT CORRECTLY, DONT COPY OTHER SOLUTIONS. SOLVE ALL PARTS. WRITE DOWN ALL OF YOUR ASSUMPTIONS. Remember that Mixture can be expressed as sum of partial molar heat capacities.
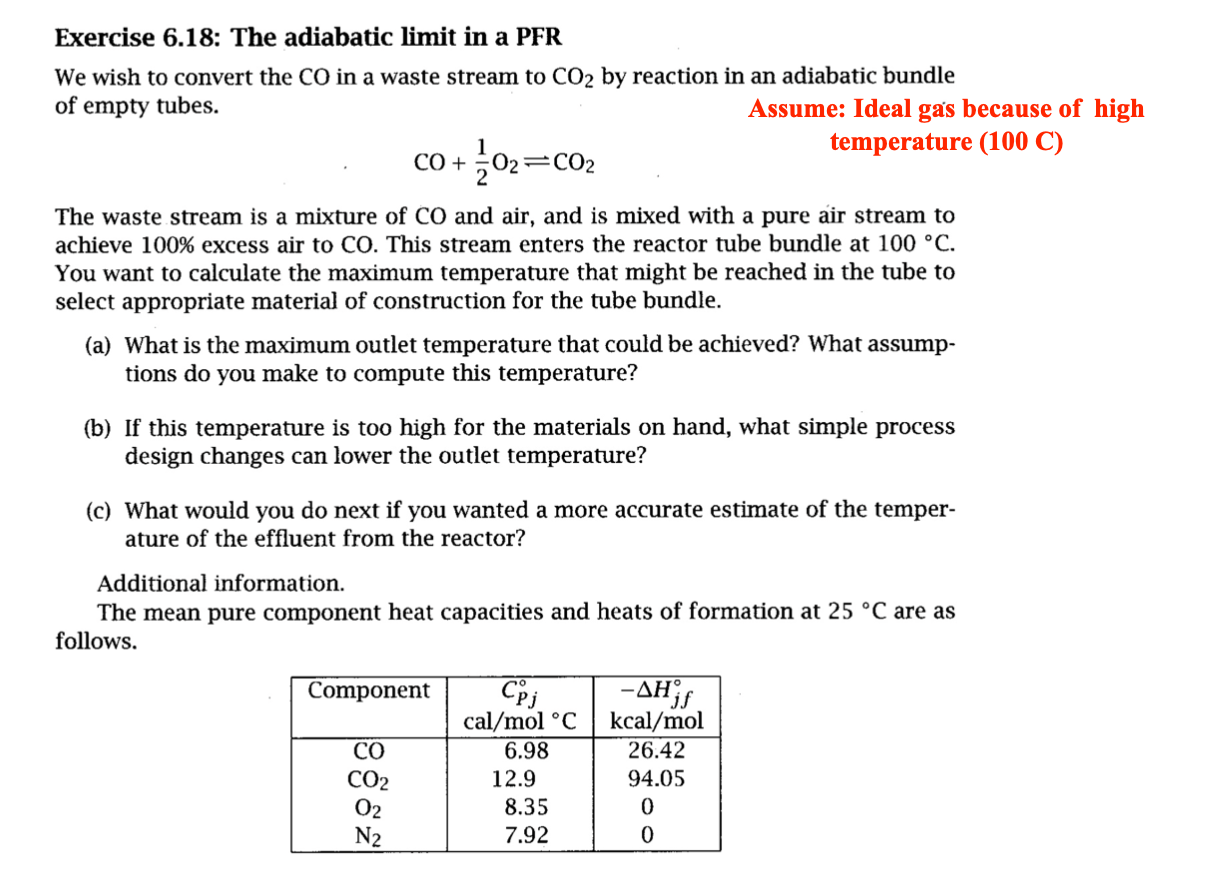
Exercise 6.18: The adiabatic limit in a PFR We wish to convert the CO in a waste stream to CO2 by reaction in an adiabatic bundle of empty tubes. Assume: Ideal gas because of high CO+21O2CO2 temperature (100C) The waste stream is a mixture of CO and air, and is mixed with a pure air stream to achieve 100% excess air to CO. This stream enters the reactor tube bundle at 100C. You want to calculate the maximum temperature that might be reached in the tube to select appropriate material of construction for the tube bundle. (a) What is the maximum outlet temperature that could be achieved? What assumptions do you make to compute this temperature? (b) If this temperature is too high for the materials on hand, what simple process design changes can lower the outlet temperature? (c) What would you do next if you wanted a more accurate estimate of the temperature of the effluent from the reactor? Additional information. The mean pure component heat capacities and heats of formation at 25C are as follows
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


