Question: Let us consider Joule heating experiment (see also Fig.2). Let us take an experimental data set in Fig.2. The experimental setting is the following;
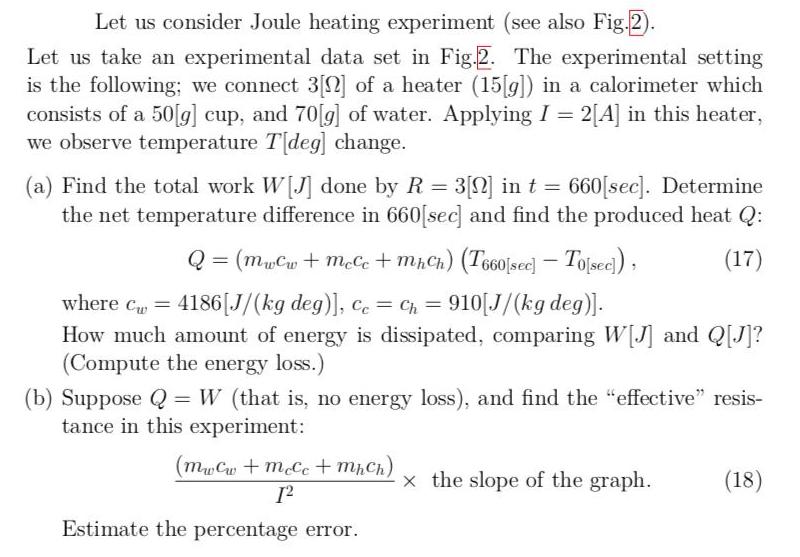
![Figure 2: A data set and its fit from Joule heating experiment. temperature [deg] v.s. time [sec] 0.0321*x + 23.7 50.0 time [](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2021/12/61acc7ff128c2_1638713341485.jpg)
Let us consider Joule heating experiment (see also Fig.2). Let us take an experimental data set in Fig.2. The experimental setting is the following; we connect 3[N] of a heater (15[g]) in a calorimeter which consists of a 50[g] cup, and 70[g] of water. Applying I = 2[A] in this heater, we observe temperature T[deg] change. (a) Find the total work W[J] done by R = 3[S] in t = 660[sec]. Determine the net temperature difference in 660[sec] and find the produced heat Q: %3D Q = (muCw + meCe + mhCh) (T660[scec) Tojsec) , (17) where Cu 4186[J/(kg deg)], cc = Ch = 910[J/(kg deg)]. %3D %3D How much amount of energy is dissipated, comparing W[J] and Q[J]? (Compute the energy loss.) (b) Suppose Q = W (that is, no energy loss), and find the "effective" resis- tance in this experiment: (MwCw +mcCc+ mhCh) x the slope of the graph. (18) Estimate the percentage error. Figure 2: A data set and its fit from Joule heating experiment. time [sec] temperature [deg] temperature [deg] v.s. time [sec] 24.4 60 25.6 0.0321*x + 23.7 120 27.2 50.0 180 29.4 240 31.5 300 32.8 360 34.7 40.0 420 36.8 480 39.0 540 41.1 600 43.2 30.0 660 45.2 20.0 200 400 600
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it
Solutin a Alork Done W IRt where I2Amp R 32 t 660 sec W 2 22 3x 660 ... View full answer

Get step-by-step solutions from verified subject matter experts


