Question: Let's check the phone application buffers for it's ability to calculate how to prepare a phosphate buffer. Suppose I want 1 liter of a 0
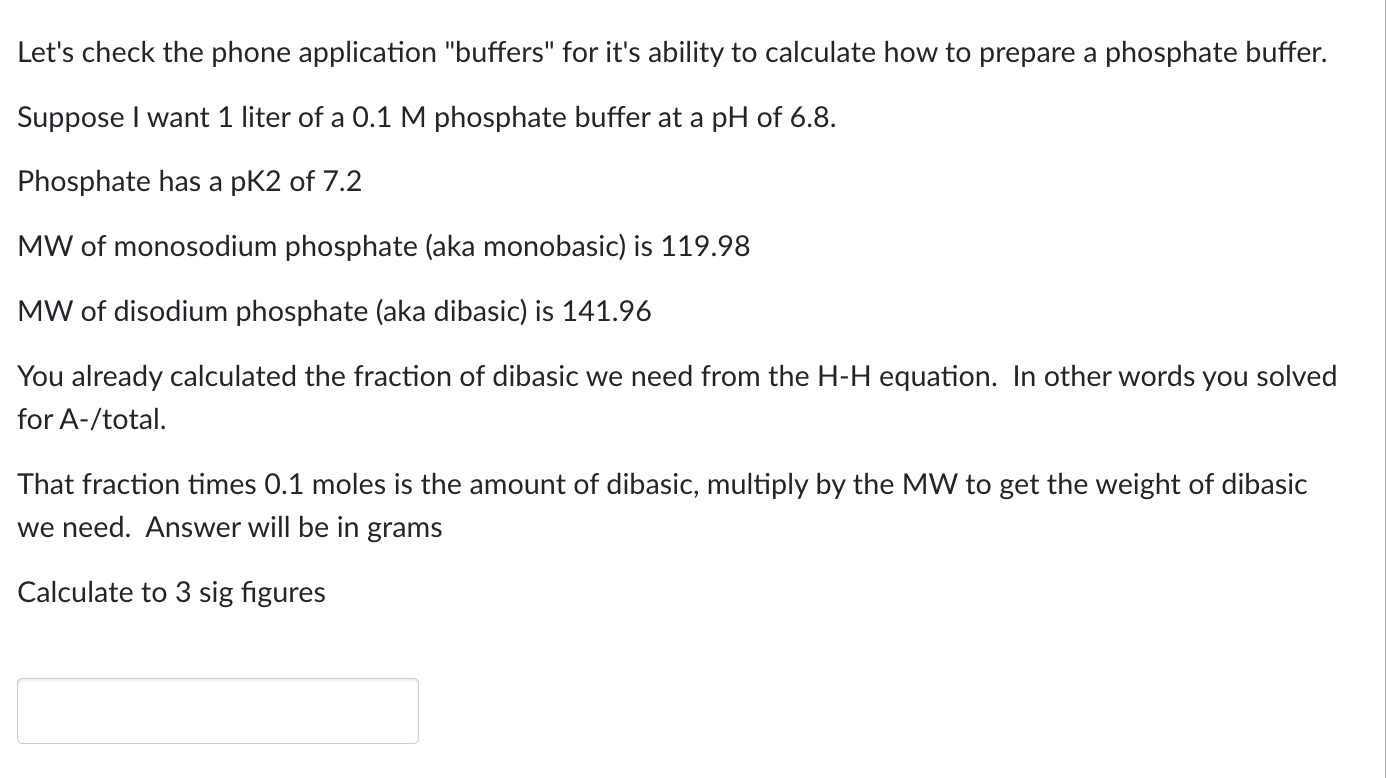
Let's check the phone application "buffers" for it's ability to calculate how to prepare a phosphate buffer.
Suppose I want liter of a phosphate buffer at a pH of
Phosphate has a pK of
MW of monosodium phosphate aka monobasic is
MW of disodium phosphate aka dibasic is
You already calculated the fraction of dibasic we need from the equation. In other words you solved
for Atotal
That fraction times moles is the amount of dibasic, multiply by the MW to get the weight of dibasic
we need. Answer will be in grams
Calculate to sig figures
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


