Question: . Let's consider spherical micelles in an aqueous solution comprised of a positively charged surfactant. Each surfactant molecule has a +le charge, where e is
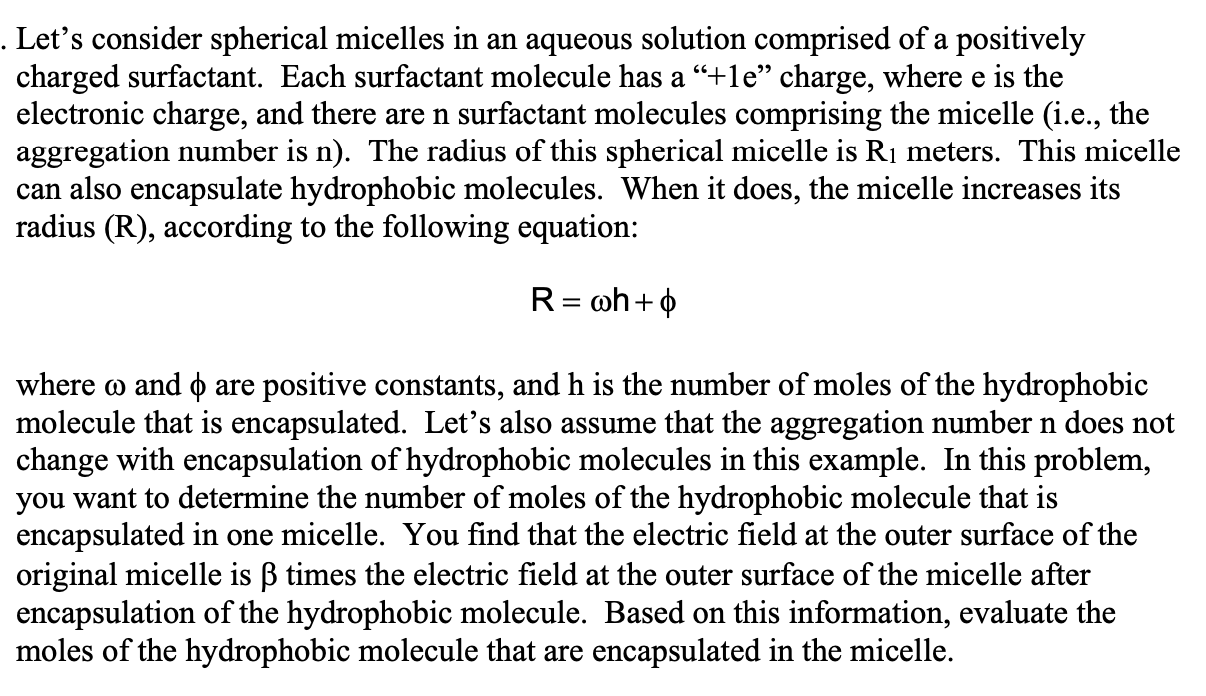
. Let's consider spherical micelles in an aqueous solution comprised of a positively charged surfactant. Each surfactant molecule has a +le charge, where e is the electronic charge, and there are n surfactant molecules comprising the micelle (i.e., the aggregation number is n). The radius of this spherical micelle is R1 meters. This micelle can also encapsulate hydrophobic molecules. When it does, the micelle increases its radius (R), according to the following equation: R= wh+ == where w and 0 are positive constants, and h is the number of moles of the hydrophobic molecule that is encapsulated. Lets also assume that the aggregation number n does not change with encapsulation of hydrophobic molecules in this ex ple. In this problem, you want to determine the number of moles of the hydrophobic molecule that is encapsulated in one micelle. You find that the electric field at the outer surface of the original micelle is times the electric field at the outer surface of the micelle after encapsulation of the hydrophobic molecule. Based on this information, evaluate the moles of the hydrophobic molecule that are encapsulated in the micelle. . Let's consider spherical micelles in an aqueous solution comprised of a positively charged surfactant. Each surfactant molecule has a +le charge, where e is the electronic charge, and there are n surfactant molecules comprising the micelle (i.e., the aggregation number is n). The radius of this spherical micelle is R1 meters. This micelle can also encapsulate hydrophobic molecules. When it does, the micelle increases its radius (R), according to the following equation: R= wh+ == where w and 0 are positive constants, and h is the number of moles of the hydrophobic molecule that is encapsulated. Lets also assume that the aggregation number n does not change with encapsulation of hydrophobic molecules in this ex ple. In this problem, you want to determine the number of moles of the hydrophobic molecule that is encapsulated in one micelle. You find that the electric field at the outer surface of the original micelle is times the electric field at the outer surface of the micelle after encapsulation of the hydrophobic molecule. Based on this information, evaluate the moles of the hydrophobic molecule that are encapsulated in the micelle
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


