Question: Let's derive the General Rate Law for the Bergman cyclization of 1,2-diethynylbenzene (we will use this specific substrate for a number of exercises this semester).
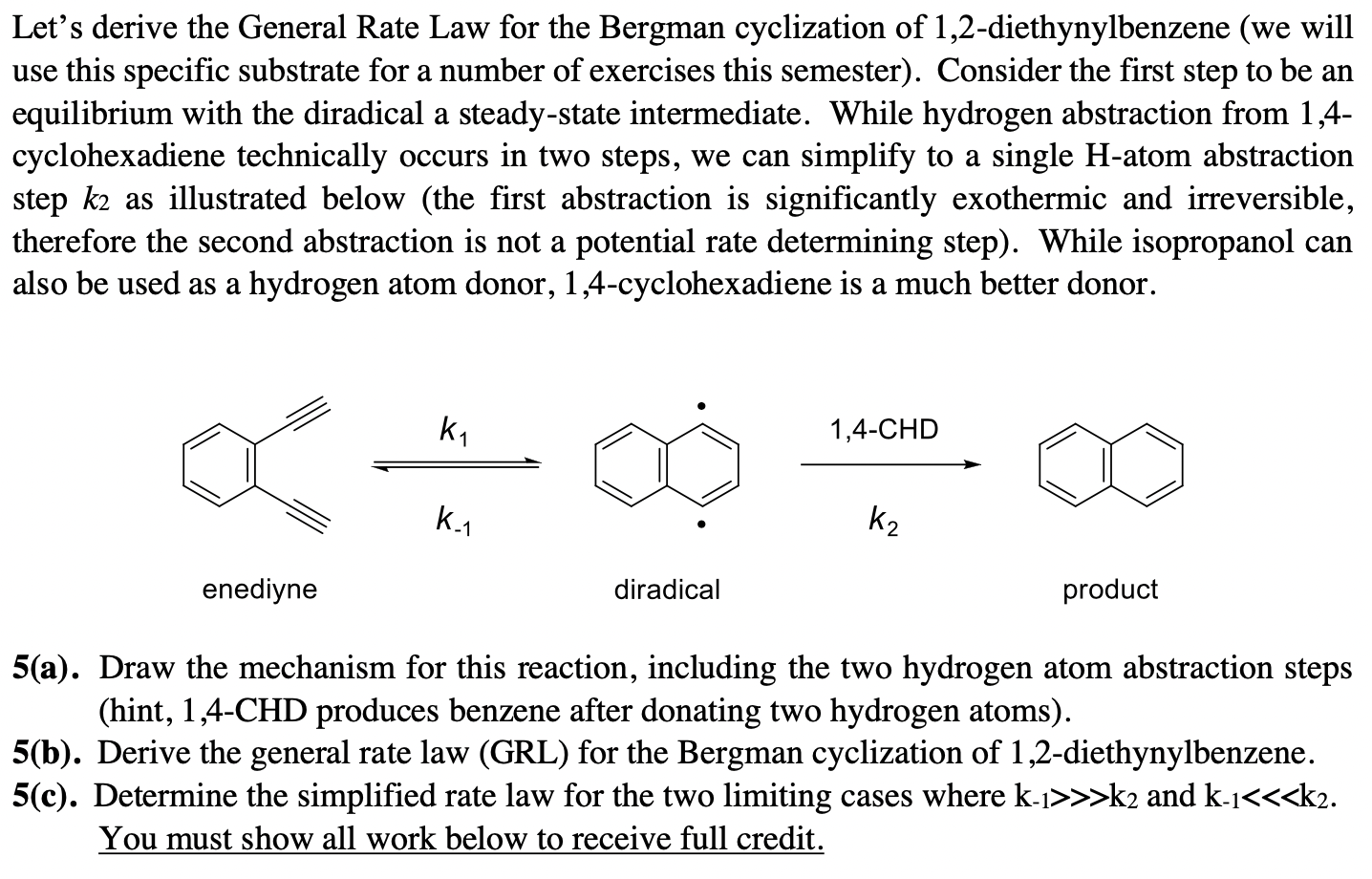
Let's derive the General Rate Law for the Bergman cyclization of 1,2-diethynylbenzene (we will use this specific substrate for a number of exercises this semester). Consider the first step to be an equilibrium with the diradical a steady-state intermediate. While hydrogen abstraction from 1,4cyclohexadiene technically occurs in two steps, we can simplify to a single H-atom abstraction step k2 as illustrated below (the first abstraction is significantly exothermic and irreversible, therefore the second abstraction is not a potential rate determining step). While isopropanol can also be used as a hydrogen atom donor, 1,4-cyclohexadiene is a much better donor. k21,4CHD enediyne diradical product 5(a). Draw the mechanism for this reaction, including the two hydrogen atom abstraction steps (hint, 1,4-CHD produces benzene after donating two hydrogen atoms). 5(b). Derive the general rate law (GRL) for the Bergman cyclization of 1,2-diethynylbenzene. 5(c). Determine the simplified rate law for the two limiting cases where k1>>k2 and k1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


