Question: Let's show why copper is that reddish color. (a) Copper has a partially filled valence band and a band gap. Draw the band structure
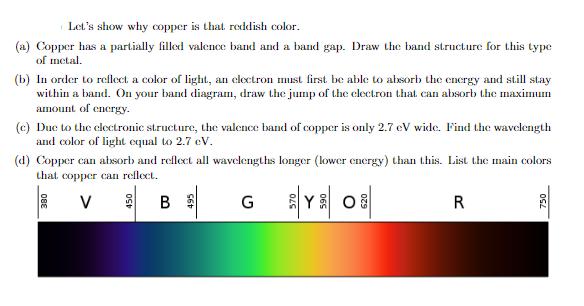
Let's show why copper is that reddish color. (a) Copper has a partially filled valence band and a band gap. Draw the band structure for this type of metal. (b) In order to reflect a color of light, an electron must first be able to absorb the energy and still stay within a band. On your band diagram, draw the jump of the electron that can absorb the maximum amount of energy. (c) Due to the electronic structure, the valence band of copper is only 2.7 eV wide. Find the wavelength and color of light equal to 2.7 eV. (d) Copper can absorb and reflect all wavelengths longer (lower energy) than this. List the main colors that copper can reflect. V Y 08 380 B G R 750
Step by Step Solution
There are 3 Steps involved in it
a Copper is a metal so it does not have a band gap Instead it has a partia... View full answer

Get step-by-step solutions from verified subject matter experts


