Question: Lewis Dot Structures/Bonding Patterns/Line Angle Drawings: 1) What are the bonding patterns (how many bonds and lone pairs) for the following atoms: a) B,C,N,O,F 2)
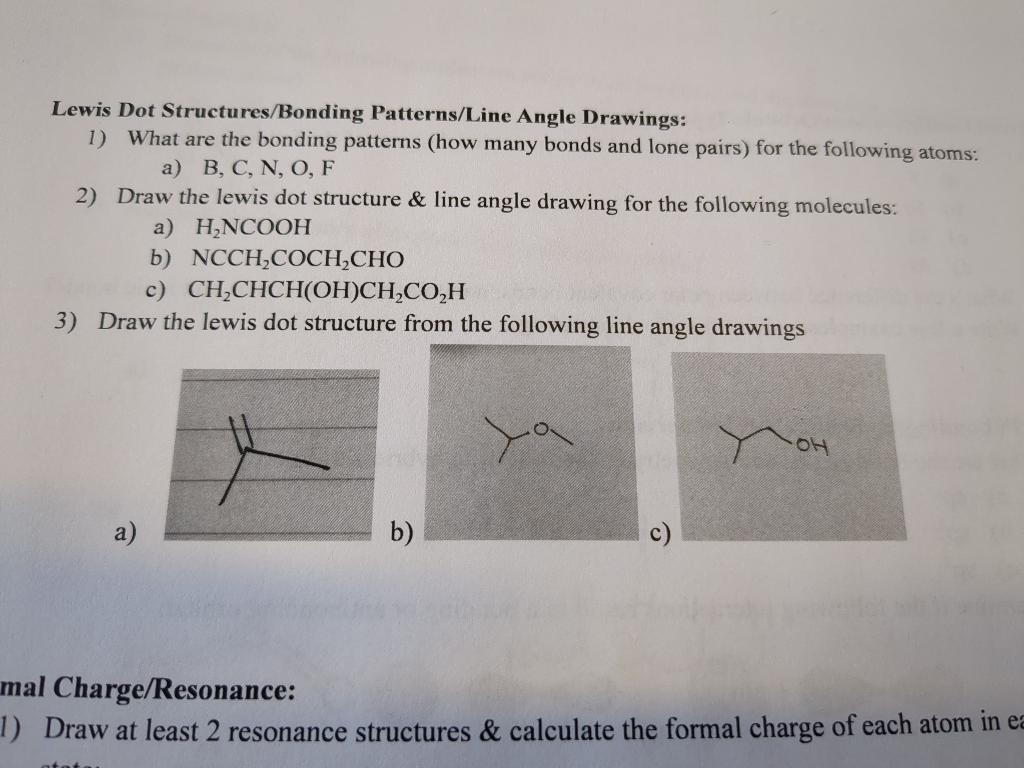
Lewis Dot Structures/Bonding Patterns/Line Angle Drawings: 1) What are the bonding patterns (how many bonds and lone pairs) for the following atoms: a) B,C,N,O,F 2) Draw the lewis dot structure \& line angle drawing for the following molecules: a) H2NCOOH b) NCCH2COCH2CHO c) CH2CHCH(OH)CH2CO2H 3) Draw the lewis dot structure from the following line angle drawings a) b) c) mal Charge/Resonance: Draw at least 2 resonance structures \& calculate the formal charge of each atom in e
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


