Question: Liguld octane (CH (CH) CH) will react with gaseous onypen (0) to produce gaseous carbon dioxide (CO) and pawon water (110) Suppose 5.71 got octane
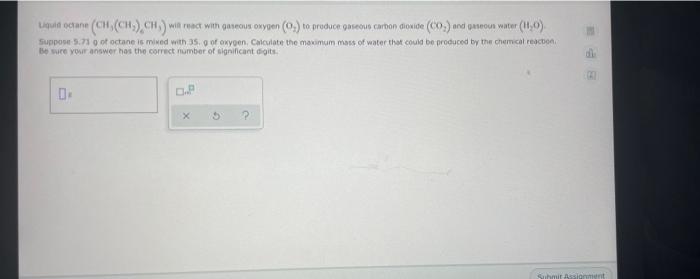
Liguld octane (CH (CH) CH) will react with gaseous onypen (0) to produce gaseous carbon dioxide (CO) and pawon water (110) Suppose 5.71 got octane is mixed with 35 g of oxygen Calculate the maximum mass of water that could be produced by the chemical reaction Be sure your answer has the correct number of significant digits dil 1.P X ? Submit it
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


