Question: Link: https://youtu.be/abec The link of the lab open in you tube and he is doing experiment like reset button and other things for you in

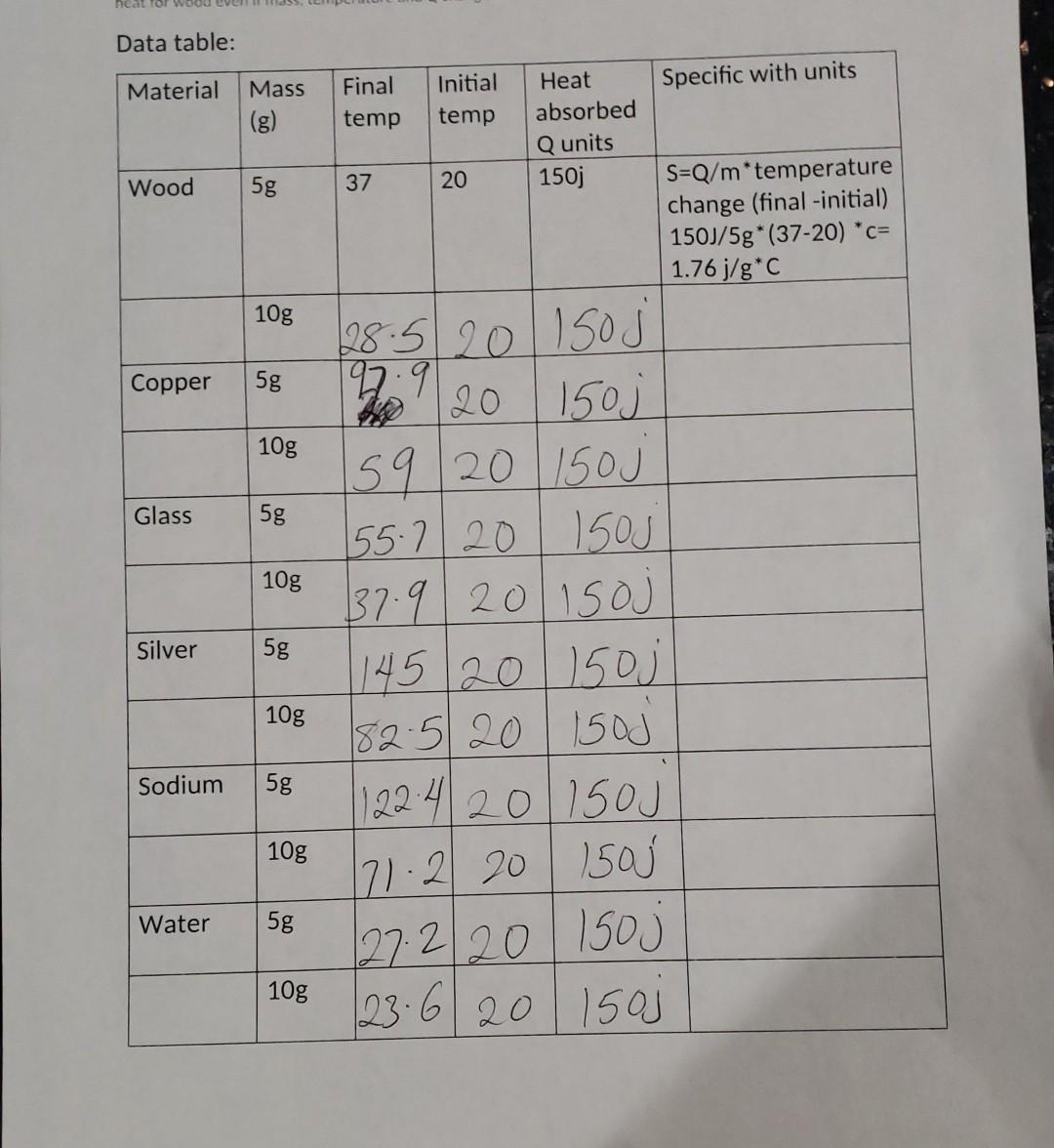
Link: https://youtu.be/abec The link of the lab open in you tube and he is doing experiment like reset button and other things for you in the vedios. You need to record the data from the vedio as per material. I did one recording for you and you can watch and fill other recording remember amount of heat he is using is almost constant which 150 J. You need to calculate specific heat capacity of each substance. Formula: Specific heat = Q/m * (final-initial temperature) Make sure to solve bottom first and divide by Q Steps which he is following in vedios: 1. Choose the substance from the material drop down menu one by one. 2. Choose the mass of the block first 5g and then 10g 3. Set the flame duration for three second (3sec for all of them) 4. Heat it. Note initial and final temp in your chart below after flame stop. 5. Record the heat absorbed or added for each substance with unit which is 150 Joules. 6. From data you got calculate specific of substance using formula. 7. Reset it and repeat step 1 to 6 for next material with same setting Please complete data table below and show your calculations on separate piece of paper. Do not worry about rounding issue just take two digits after decimal in your calculation neat for wood ever Data table: Mass Material Specific with units Final temp Initial temp Heat absorbed Q units 150j Wood 5g 37 20 S=Q/m*temperature change (final -initial) 150J/5g* (37-20) *c= 1.76j/g*C 10g Copper 5g 10g Glass 5g 10g 28.5 20 150J 197:9 20 150 159/20 150J 155.7 20 150J 137.920 150J 14520 15oj 182.520 1500 1122 4201150J Silver 5g 10g Sodium 5g 10g Water 5g 171.2 201501 27.220 Isoj 108 [23-6-201503
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


