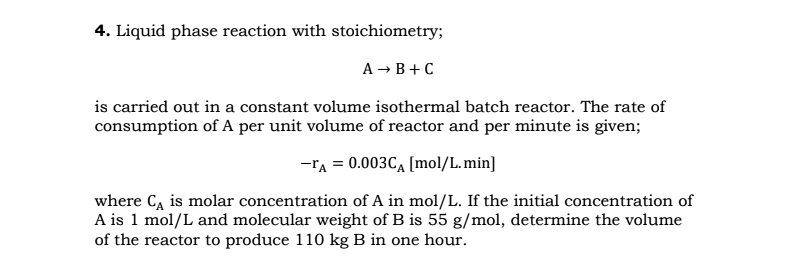
Question: Liquid phase reaction with stoichiometry; A B + C is carried out in a constant volume isothermal batch reactor. The rate of consumption of A
Liquid phase reaction with stoichiometry;
is carried out in a constant volume isothermal batch reactor. The rate of
consumption of A per unit volume of reactor and per minute is given;
where is molar concentration of in If the initial concentration of
is and molecular weight of is determine the volume
of the reactor to produce in one hour.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


