Question: LQ 4. Consider the table below and answer the questions that follow. A Propan-1-ol B th H . H H OH H H CI H
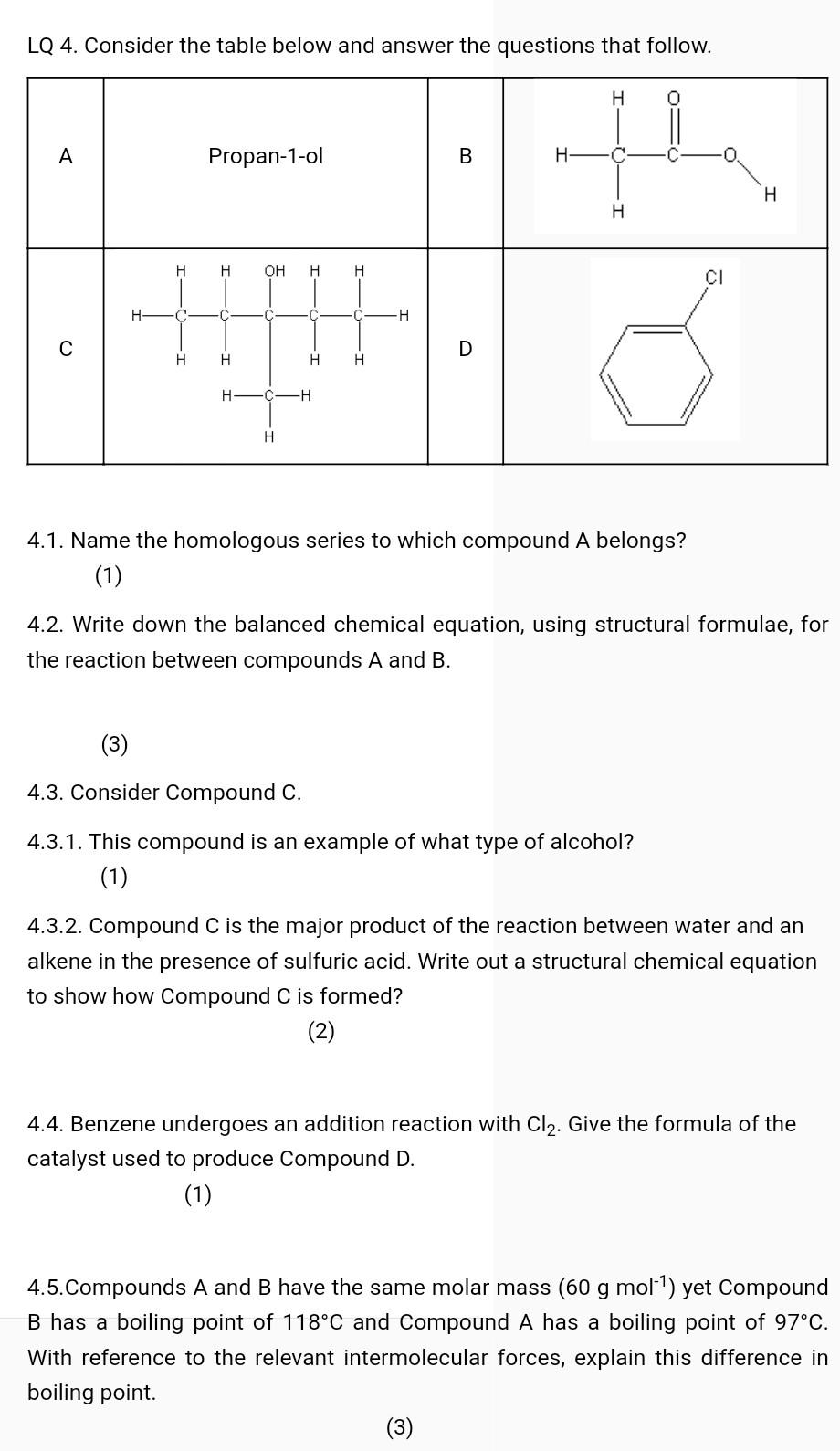
LQ 4. Consider the table below and answer the questions that follow. A Propan-1-ol B th H . H H OH H H CI H C C -H C D H H H H - -H H 4.1. Name the homologous series to which compound A belongs? (1) 4.2. Write down the balanced chemical equation, using structural formulae, for the reaction between compounds A and B. (3) 4.3. Consider Compound C. 4.3.1. This compound is an example of what type of alcohol? (1) 4.3.2. Compound C is the major product of the reaction between water and an alkene in the presence of sulfuric acid. Write out a structural chemical equation to show how Compound C is formed? (2) 4.4. Benzene undergoes an addition reaction with Cl2. Give the formula of the catalyst used to produce Compound D. (1) 4.5.Compounds A and B have the same molar mass (60 g mol-) yet Compound B has a boiling point of 118C and Compound A has a boiling point of 97C. With reference to the relevant intermolecular forces, explain this difference in boiling point. (3)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


