Question: m = 0 . 1 2 7 k g g 2 when P V = M R T tube has a diameter of 2 5
when
tube has a diameter of in determine its length pressure absolute contains of sonitad.
a Assuming meth
b Using the compane follows the ideal gas model
mpressibility chart
of methane. If the
Properties of methane gas cal gas model is a good fit for methane at these conditions. kPa
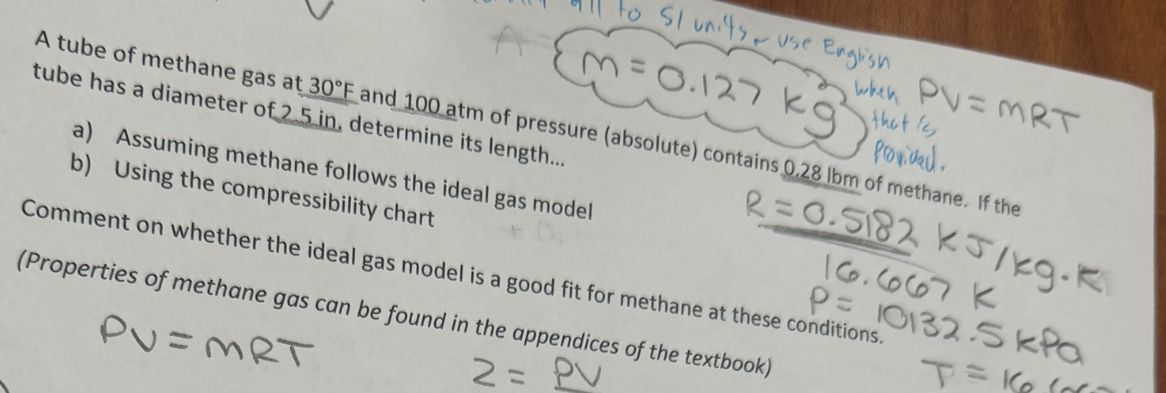
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


