Question: M in F. pH after addition pH before addition =pH change = A 22.2mL sample of 0.339M ethylamine, C2H5NH2, is titrated with 0.318M perchloric acid.
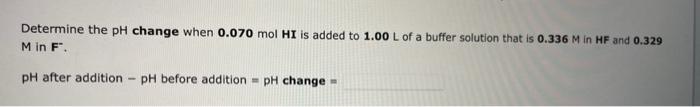

M in F. pH after addition pH before addition =pH change = A 22.2mL sample of 0.339M ethylamine, C2H5NH2, is titrated with 0.318M perchloric acid. After adding 9.96mL of perchloric acid, the pH is Use the Tables link in the References for any equilibrium constants that are required
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


