Question: Because of side reactions, the actual yield of a product is often less than the theoretical yield. The percent yield is defined as: %
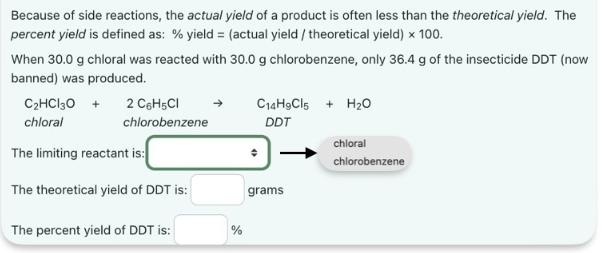
Because of side reactions, the actual yield of a product is often less than the theoretical yield. The percent yield is defined as: % yield = (actual yield / theoretical yield) x 100. When 30.0 g chloral was reacted with 30.0 g chlorobenzene, only 36.4 g of the insecticide DDT (now banned) was produced. C14H9C15 + H2O CHCl30 + chloral 2 C6H5Cl chlorobenzene DDT The limiting reactant is: The theoretical yield of DDT is: The percent yield of DDT is: % grams chloral chlorobenzene
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


