Question: make observations about the data 37 1H NMR:615 (6H, m), 2.144H, m, 4.5/2Hs) secesionary nyte C1H NMR: 61.5 (3H, sl. 1.5 2.118H, m) 4.51HS) TASK:
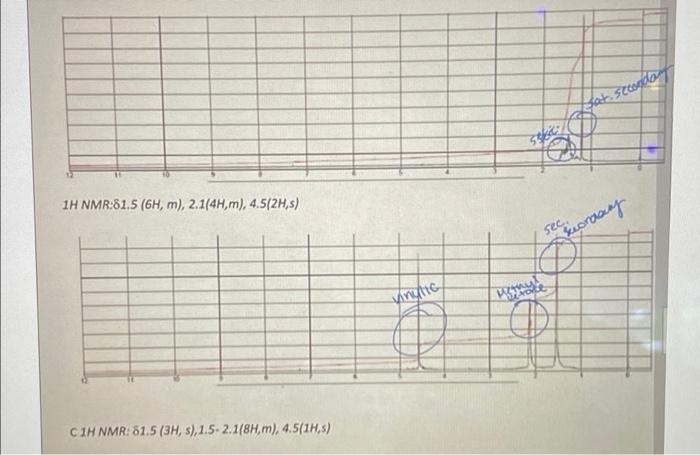

37 1H NMR:615 (6H, m), 2.144H, m, 4.5/2Hs) secesionary nyte C1H NMR: 61.5 (3H, sl. 1.5 2.118H, m) 4.51HS) TASK: Your friend followed the procedure given below to carry out the following synthesis reaction in the lab. This is very similar to the elimination lab you ran in our curriculum. DEHYDRATION OF AN ALCOHOL conc. H2SO4 OH You Chemical Formula: C,H140 Chemical Formula: CyH12 Molecular Weight: 114.1855 g/mol Molecular Weight: 96.1702 g/mol As a part of the scientific communication learning outcome for this course, your friend has to show a plan and design and scientifically communicate the findings and interpretations in a lab report. He/She doesn't know where to begin. Fortunately, you already have some experience to write down a lab journal. Help your friend devise. Provide a process map (3 points) and write a formally written lab with full data analysis - AIM (1 point), materials (1 point), equation and mechanism (2point), procedure (2point)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


