Question: Making an ICE table A flask is filled with Fe3+ and SCN, which decomposes according to the following reaction Fe3+(aq)+SCN(aq)[FeSCN2+](aq) At the very beginning of
 At the very](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8d3e0cd0b7_20066f8d3e067cb3.jpg)
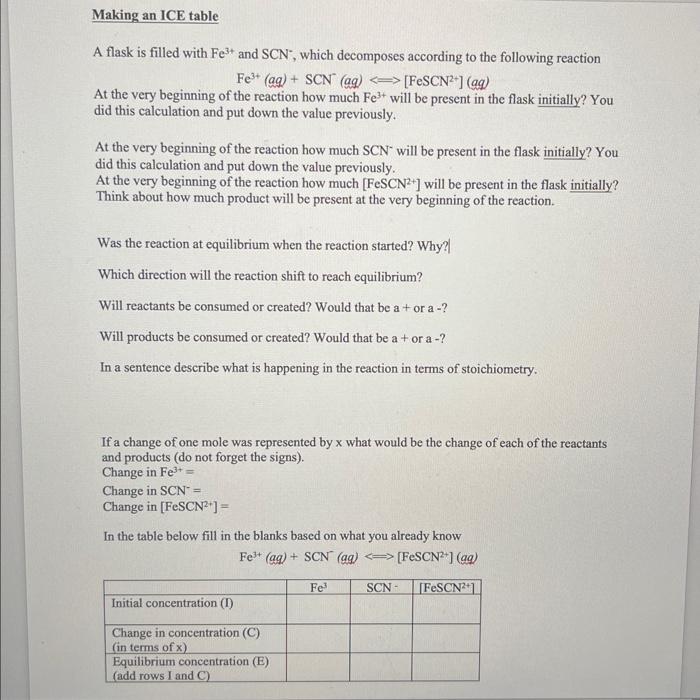
Making an ICE table A flask is filled with Fe3+ and SCN, which decomposes according to the following reaction Fe3+(aq)+SCN(aq)[FeSCN2+](aq) At the very beginning of the reaction how much Fe3+ will be present in the flask initially? You did this calculation and put down the value previously. At the very beginning of the reaction how much SCNwill be present in the flask initially? You did this calculation and put down the value previously. At the very beginning of the reaction how much [FeSCN2+ ] will be present in the flask initially? Think about how much product will be present at the very beginning of the reaction. Was the reaction at equilibrium when the reaction started? Why?| Which direction will the reaction shift to reach equilibrium? Will reactants be consumed or created? Would that be a + or a ? Will products be consumed or created? Would that be a + or a ? In a sentence describe what is happening in the reaction in terms of stoichiometry. If a change of one mole was represented by x what would be the change of each of the reactants and products (do not forget the signs). Change in Fe3+= Change in SCN= Change in [FeSCN2+]= In the table below fill in the blanks based on what you already know Fe3+(aq)+SCN(ag)[FeSCN2+](ag) We know the value of x because this is the concentration of [FeSCN2+] which you calculated from the graph. So you can use this to calculate the value of x and therefore the equilibrium concentrations of Fe3+ and SCN. SHOW ALL YOUR WORK! [Fe3+]eq= [SCN]eq= [FeSCN2+]eq= Now that you have these equilibrium concentrations (values) plug them into the equilibrium constant expression to calculate the value of Kc. SHOW ALL YOUR WORK! Kc=[Fe3+][SCN][FeSCN2+] This is the value of Kc that you found from Solution 1 Do you expect the value of Kc that you will find from Solutions 2-3 to be around this number or be different from the number you just determined? Why or why not? Justify and explain your reasoning. Before you leave the lab, you need to do the same calculation that you did for solution 1 (beginning of Part 3 on page 5. Use this worksheet as a template to do those calculations on solutions 2 and 3 and calculate an average value of Kc from all the 3 values. Make sure you show the calculation showing how you determined the average. SHOW ALL YOUR WORK
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


