Question: Many bases have a nitrogen group with a lone pair called an amine group. When methylamine reacts with water it follows the below process: CH3NH2(aq)+H2O(l)CH3NH3+(aq)+OH(aq)
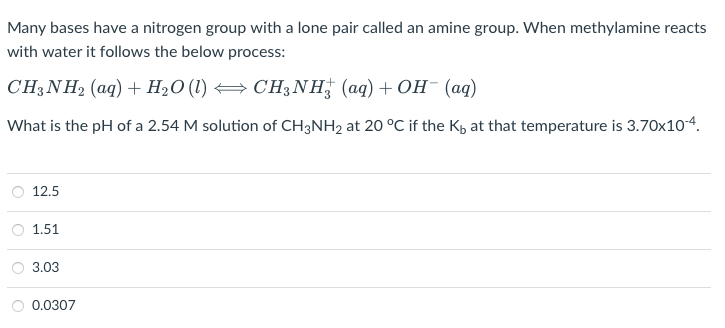
Many bases have a nitrogen group with a lone pair called an amine group. When methylamine reacts with water it follows the below process: CH3NH2(aq)+H2O(l)CH3NH3+(aq)+OH(aq) What is the pH of a 2.54M solution of CH3NH2 at 20C if the Kb at that temperature is 3.70104. \begin{tabular}{l} 12.5 \\ \hline 1.51 \\ \hline 3.03 \\ \hline 0.0307 \end{tabular}
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


