Question: Mass Transfer Ammonia gas is diffusing through nitrogen under steady-state condition with nitrogen non-diffusing since it is insoluble in one boundary. The total pressure is
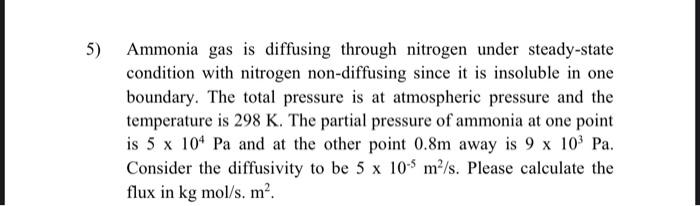
Ammonia gas is diffusing through nitrogen under steady-state condition with nitrogen non-diffusing since it is insoluble in one boundary. The total pressure is at atmospheric pressure and the temperature is 298K. The partial pressure of ammonia at one point is 5104Pa and at the other point 0.8m away is 9103Pa. Consider the diffusivity to be 5105m2/s. Please calculate the flux in kgmol/s.m2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


