Question: Match the column: Column I 10 M MgO (d solution = 1.20 gm/ml) Solute: MgO Solvent: HO 40% w/v NaOH (d solution = 1.6
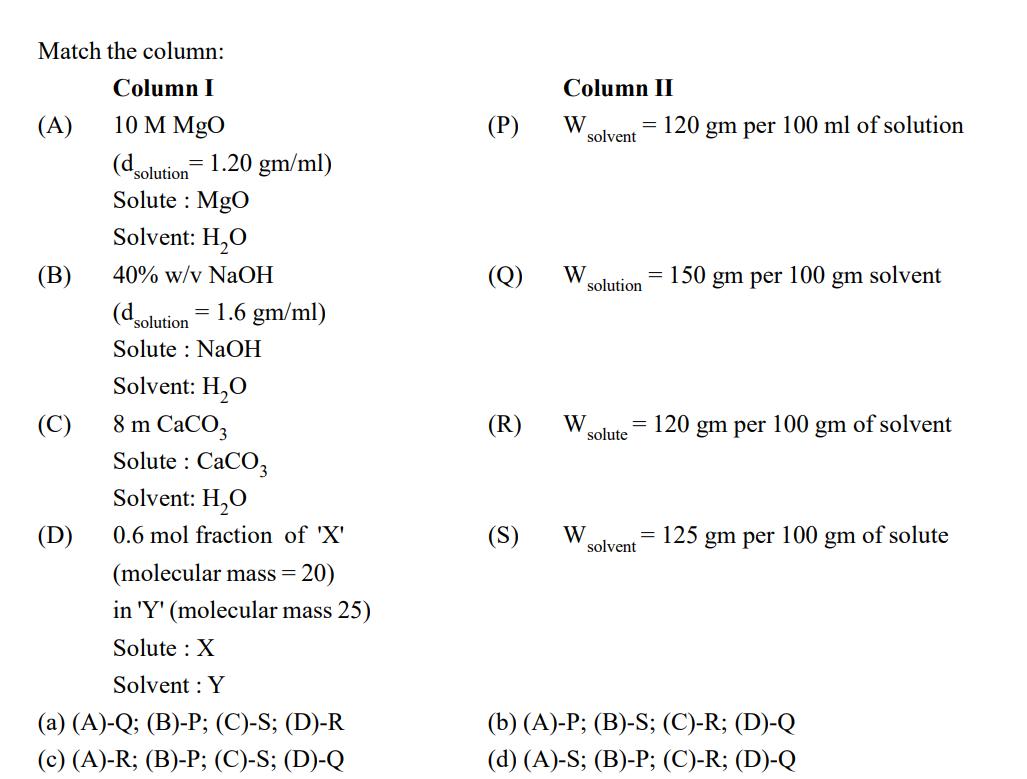
Match the column: Column I 10 M MgO (d solution = 1.20 gm/ml) Solute: MgO Solvent: HO 40% w/v NaOH (d solution = 1.6 gm/ml) Solute NaOH (A) (B) (C) (D) Solvent: HO 8 m CaCO3 Solute : CaCO3 Solvent: HO 0.6 mol fraction of 'X' (molecular mass = 20) in 'Y' (molecular mass 25) Solute : X Solvent: Y (a) (A)-Q; (B)-P; (C)-S; (D)-R (c) (A)-R; (B)-P; (C)-S; (D)-Q (P) Column II W = 120 gm per 100 ml of solution solvent (Q) W solution (S) W = = 150 gm per 100 gm solvent (R) W = solute solvent = 120 gm per = 100 gm (b) (A)-P; (B)-S; (C)-R; (D)-Q (d) (A)-S; (B)-P; (C)-R; (D)-Q of solvent 125 gm per 100 gm of solute
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


